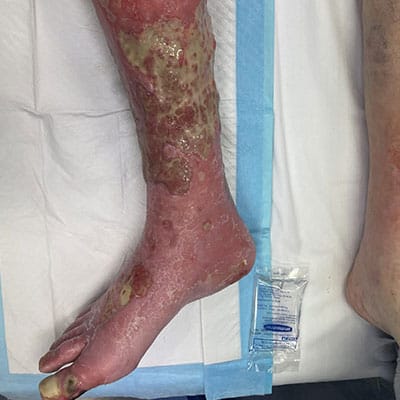
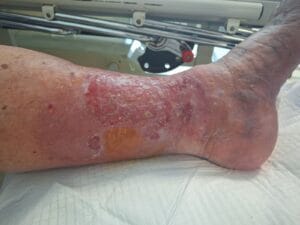
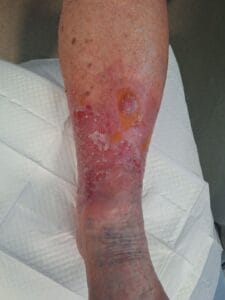
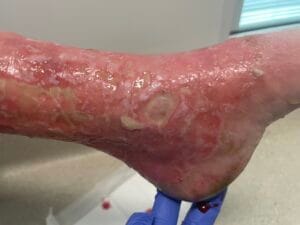
Associated References
Main Article
Aetiology
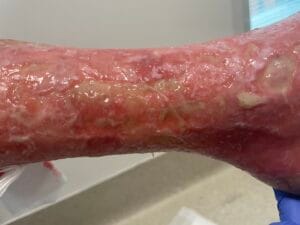
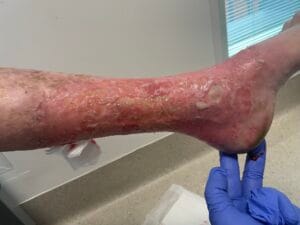
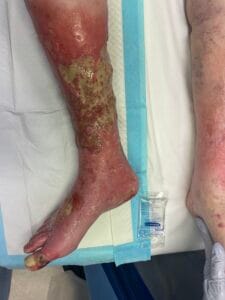
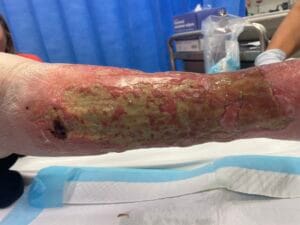
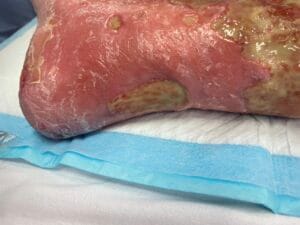
Venous leg ulcers (VLUs) are primarily caused by chronic venous insufficiency (CVI), which results from the failure of venous valves and impaired venous return. This condition leads to sustained venous hypertension, causing damage to the skin and underlying tissues, ultimately resulting in ulceration (Phillips et al., 2017; Bland et al., 2015). The pathophysiological mechanisms involve a complex interplay of factors, including venous reflux, obstruction, and calf muscle pump dysfunction (Phillips et al., 2017; Bland et al., 2015). Additionally, conditions such as obesity, age, and a history of deep vein thrombosis can exacerbate venous insufficiency, increasing the risk of ulcer formation (Probst et al., 2019; Rice et al., 2014).
The inflammatory response triggered by venous hypertension plays a critical role in the development of VLUs. Prolonged venous stasis leads to the release of inflammatory mediators, which can cause tissue damage and further exacerbate the ulceration process (Engbers et al., 2015; Raffetto, 2014). Furthermore, the presence of comorbidities such as diabetes and peripheral arterial disease can complicate the healing process, making it essential for clinicians to understand the multifactorial aetiology of VLUs to provide effective management (DeBacker et al., 2021).
Prevalence
The prevalence of venous leg ulcers is significant, particularly in older populations. Recent studies indicate that VLUs affect approximately 1% to 3% of the general population in industrialized countries, with higher rates observed in individuals over 60 years of age (Probst et al., 2019; Rice et al., 2014). In the United States, the incidence of VLUs is estimated to be around 2.2% among Medicare beneficiaries, highlighting the substantial burden of this condition on healthcare systems (Nussbaum et al., 2018). Furthermore, VLUs account for up to 90% of all lower extremity ulcers, underscoring their prominence as a chronic wound type (Rice et al., 2014).
The economic impact of VLUs is also considerable, with treatment costs reaching billions annually. For instance, it is estimated that the cost of managing VLUs in the U.S. alone is approximately $3 billion per year, reflecting the need for effective prevention and treatment strategies (Raffetto, 2014). Given these statistics, it is crucial for healthcare professionals to be aware of the prevalence and economic burden associated with VLUs to advocate for appropriate resource allocation and patient care initiatives.
Pathophysiological Changes
The pathophysiological changes associated with venous leg ulcers are primarily driven by chronic venous hypertension, which leads to a cascade of inflammatory and cellular responses. The sustained pressure in the venous system causes endothelial dysfunction, resulting in increased permeability and the extravasation of plasma proteins into the interstitial space (Engbers et al., 2015; Raffetto, 2014). This process contributes to the formation of edema, which further compromises tissue oxygenation and nutrient delivery, creating an environment conducive to ulceration (Engbers et al., 2015; Raffetto, 2014).
In addition to edema, the inflammatory response plays a pivotal role in the development of VLUs. The release of pro-inflammatory cytokines and growth factors leads to the activation of fibroblasts and keratinocytes, which are essential for wound healing (Aschermann et al., 2017). However, in the context of chronic venous insufficiency, this response becomes dysregulated, resulting in impaired wound healing and the persistence of ulcers (Engbers et al., 2015; Aschermann et al., 2017). Moreover, the presence of biofilm-forming bacteria in chronic wounds can exacerbate inflammation and delay healing, necessitating a comprehensive understanding of the microbiological aspects of VLUs (Engbers et al., 2015; Aschermann et al., 2017).
Treatments
Current evidence-based treatment options for venous leg ulcers primarily focus on addressing the underlying venous insufficiency and promoting wound healing. Compression therapy remains the cornerstone of VLU management, as it aids in reducing venous hypertension and improving venous return (Dissemond et al., 2016; Andriessen et al., 2017). Various forms of compression, including bandages and stockings, have been shown to enhance healing rates significantly (Dissemond et al., 2016; Andriessen et al., 2017).
In addition to compression, advanced wound dressings play a crucial role in managing VLUs. These dressings help maintain a moist wound environment, promote autolytic debridement, and protect against infection (Serra et al., 2016; Serena et al., 2014). Recent studies have also explored the use of biological therapies, such as dehydrated human amnion/chorion membrane allografts, which have demonstrated promising results in enhancing healing outcomes when combined with compression therapy (Serena et al., 2014).
Surgical interventions, including endovenous ablation and sclerotherapy, have emerged as effective adjuncts to conservative management, particularly in patients with significant superficial venous reflux (Gohel et al., 2018; Tollow & Ogden, 2016). These minimally invasive procedures aim to address the underlying venous pathology, thereby reducing the risk of ulcer recurrence and improving overall patient outcomes (Gohel et al., 2018; Tollow & Ogden, 2016).
Precautions with Treatments
While numerous treatment options exist for VLUs, clinicians must be aware of specific precautions and contraindications associated with these interventions. For instance, compression therapy is contraindicated in patients with arterial insufficiency, as it may exacerbate ischemia and lead to further complications (Andriessen et al., 2017). Therefore, it is essential to assess the Ankle-Brachial Pressure Index (ABPI) prior to initiating compression therapy to ensure patient safety (Andriessen et al., 2017).
Additionally, the use of advanced wound dressings should be tailored to the individual patient’s needs, considering factors such as the stage of healing, presence of infection, and patient comfort (Serra et al., 2016; Serena et al., 2014). Clinicians must also be vigilant in monitoring for potential adverse effects associated with surgical interventions, including thrombosis and infection, to mitigate risks and ensure optimal healing outcomes (Gohel et al., 2018).
Diagnostic Tests Available
Accurate diagnosis of venous leg ulcers is critical for effective management. Clinicians typically employ a combination of clinical assessment and diagnostic tests to confirm the diagnosis of VLUs. The clinical evaluation includes a thorough history and physical examination, focusing on the characteristics of the ulcer, surrounding skin, and patient comorbidities (DeBacker et al., 2021).
Doppler ultrasound is a key diagnostic tool used to assess venous function and identify underlying venous reflux or obstruction (DeBacker et al., 2021). This non-invasive imaging technique allows clinicians to visualize the venous anatomy and determine the appropriate treatment strategy. Additionally, the use of the ABPI is essential in differentiating venous ulcers from arterial ulcers, guiding treatment decisions and ensuring patient safety (Andriessen et al., 2017).
In some cases, advanced imaging modalities, such as venography or magnetic resonance imaging (MRI), may be warranted to evaluate complex venous anatomy or assess for deep vein thrombosis (DeBacker et al., 2021). Overall, a comprehensive diagnostic approach is crucial for accurate identification and effective management of venous leg ulcers.
Contributing Factors
Several modifiable and non-modifiable factors contribute to the development of venous leg ulcers. Non-modifiable factors include age, gender, and genetic predisposition, with older adults and women being at higher risk for developing VLUs (Probst et al., 2019). Conversely, modifiable factors encompass lifestyle choices and comorbid conditions, such as obesity, sedentary behavior, and diabetes, which can exacerbate venous insufficiency and increase the likelihood of ulcer formation (Probst et al., 2019; Rice et al., 2014).
Addressing modifiable risk factors through patient education and lifestyle interventions is essential for preventing VLUs. Encouraging regular physical activity, weight management, and smoking cessation can significantly reduce the risk of developing venous insufficiency and associated ulcers (O’Brien et al., 2014). Furthermore, healthcare professionals should emphasize the importance of early intervention and management of comorbid conditions to mitigate the risk of ulcer development and improve patient outcomes (O’Brien et al., 2014).
Conclusion
In conclusion, venous leg ulcers represent a significant clinical challenge for healthcare professionals, necessitating a comprehensive understanding of their aetiology, prevalence, pathophysiological changes, treatment options, precautions, diagnostic methods, and contributing factors. By equipping clinicians with this knowledge, we can enhance patient care, improve healing outcomes, and reduce the burden of VLUs on healthcare systems.
References:
- Andriessen, A., Apelqvist, J., Mosti, G., Partsch, H., Gonska, C., & Abel, M. (2017). Compression therapy for venous leg ulcers: risk factors for adverse events and complications, contraindications – a review of present guidelines. Journal of the European Academy of Dermatology and Venereology, 31(9), 1562-1568. https://doi.org/10.1111/jdv.14390
- Aschermann, I., Noor, S., Venturelli, S., Sinnberg, T., Mnich, C., & Busch, C. (2017). Extracorporal shock waves activate migration, proliferation and inflammatory pathways in fibroblasts and keratinocytes, and improve wound healing in an open-label, single-arm study in patients with therapy-refractory chronic leg ulcers. Cellular Physiology and Biochemistry, 41(3), 890-906. https://doi.org/10.1159/000460503
- Bland, J., Dumville, J., Ashby, R., Gabe, R., Stubbs, N., Adderley, U., … & Cullum, N. (2015). Validation of the veines-qol quality of life instrument in venous leg ulcers: repeatability and validity study embedded in a randomised clinical trial. BMC Cardiovascular Disorders, 15(1). https://doi.org/10.1186/s12872-015-0080-7
- DeBacker, S., Bulman, J., & Weinstein, J. (2021). Wound care for venous ulceration. Seminars in Interventional Radiology, 38(02), 194-201. https://doi.org/10.1055/s-0041-1727161
- Dissemond, J., Assenheimer, B., Bültemann, A., Gerber, V., Gretener, S., Siebenthal, E., … & Partsch, H. (2016). Compression therapy in patients with venous leg ulcers. JDDG Journal Der Deutschen Dermatologischen Gesellschaft, 14(11), 1072-1087. https://doi.org/10.1111/ddg.13091
- Engbers, M., Karasu, A., Blom, J., Cushman, M., Rosendaal, F., & Vlieg, A. (2015). Clinical features of venous insufficiency and the risk of venous thrombosis in older people. British Journal of Haematology, 171(3), 417-423. https://doi.org/10.1111/bjh.13579
- Gohel, M., Heatley, F., Liu, X., Bradbury, A., Bulbulia, R., Cullum, N., … & Davies, A. (2018). A randomized trial of early endovenous ablation in venous ulceration. New England Journal of Medicine, 378(22), 2105-2114. https://doi.org/10.1056/nejmoa1801214
- Nussbaum, S., Carter, M., Fife, C., DaVanzo, J., Haught, R., Nusgart, M., … & Cartwright, D. (2018). An economic evaluation of the impact, cost, and medicare policy implications of chronic nonhealing wounds. Value in Health, 21(1), 27-32. https://doi.org/10.1016/j.jval.2017.07.007
- O’Brien, J., Finlayson, K., Kerr, G., & Edwards, H. (2014). Testing the effectiveness of a self-efficacy based exercise intervention for adults with venous leg ulcers: protocol of a randomised controlled trial. BMC Dermatology, 14(1). https://doi.org/10.1186/1471-5945-14-16
- Phillips, P., Lumley, E., Duncan, R., Aber, A., Woods, H., Jones, G., … & Michaels, J. (2017). A systematic review of qualitative research into people’s experiences of living with venous leg ulcers. Journal of Advanced Nursing, 74(3), 550-563. https://doi.org/10.1111/jan.13465
- Probst, S., Turcotte, M., & Skinner, M. (2019). Internal consistency and reliability of the swiss-french translation of the venous leg ulcer self efficacy tool (veluset). BMJ Open, 9(12), e031529. https://doi.org/10.1136/bmjopen-2019-031529
- Raffetto, J. (2014). Which dressings reduce inflammation and improve venous leg ulcer healing. Phlebology the Journal of Venous Disease, 29(1_suppl), 157-164. https://doi.org/10.1177/0268355514529225
- Rice, J., Desai, U., Cummings, A., Birnbaum, H., Skornicki, M., & Parsons, N. (2014). Burden of venous leg ulcers in the united states. Journal of Medical Economics, 17(5), 347-356. https://doi.org/10.3111/13696998.2014.903258
- Serena, T., Carter, M., Le, L., Sabo, M., & DiMarco, D. (2014). A multicenter, randomized, controlled clinical trial evaluating the use of dehydrated human amnion/chorion membrane allografts and multilayer compression therapy vs. multilayer compression therapy alone in the treatment of venous leg ulcers. Wound Repair and Regeneration, 22(6), 688-693. https://doi.org/10.1111/wrr.12227
- Serra, R., Rizzuto, A., Rossi, A., Perri, P., Barbetta, A., Abdalla, K., … & Franciscis, S. (2016). Skin grafting for the treatment of chronic leg ulcers – a systematic review in evidence‐based medicine. International Wound Journal, 14(1), 149-157. https://doi.org/10.1111/iwj.12575
- Tollow, P. and Ogden, J. (2016). Surgical management for venous leg ulcers: the role of hope, investment and agency. Journal of Health Psychology, 23(8), 1075-1084. https://doi.org/10.1177/1359105316643380