Quick Overview
Associated References
Associated Care Plans/Tutorials
Associated Products
Main Article
Silver has been utilized for its antimicrobial properties for over two millennia, with historical records indicating its use in ancient civilizations for wound treatment and infection prevention. The modern medical application of silver began in the late 19th century when Dr. B.C. Crede introduced colloidal silver for sterilizing newborns’ eyes to prevent infections (Sim et al., 2018). The use of silver gained momentum throughout the early to mid-20th century, particularly during World War I and II, where it was employed to treat battlefield wounds (Chuangsuwanich et al., 2013).
In contemporary medicine, silver is incorporated into various wound care products, including silver sulfadiazine and silver nanoparticles, which have been shown to enhance healing outcomes in chronic and acute wounds (Khansa et al., 2019). The evolution of silver-based treatments reflects ongoing research into their efficacy and safety, leading to the development of advanced formulations that leverage silver’s antimicrobial properties while minimizing potential toxicity (Nqakala et al., 2021).
Mechanism of Action
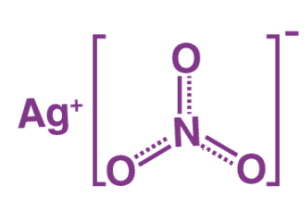
The antimicrobial action of silver is primarily attributed to the release of positively charged silver ions (Ag+), which interact with bacterial cell membranes, leading to structural alterations that inhibit cellular respiration and protein synthesis (Tsaia et al., 2014). This oligodynamic effect allows silver to exert a broad-spectrum antimicrobial action against both Gram-positive and Gram-negative bacteria, including antibiotic-resistant strains (Domínguez et al., 2020).
Silver nanoparticles (AgNPs) further enhance these properties due to their high surface area-to-volume ratio, which facilitates greater interaction with microbial cells (Mei et al., 2014). The mechanisms by which AgNPs exert their antimicrobial effects include the generation of reactive oxygen species (ROS), which induce oxidative stress in bacteria, and the disruption of cell membrane integrity (Dakal et al., 2016). Additionally, AgNPs can penetrate biofilms, which are often resistant to conventional treatments, thereby improving wound healing in chronic infections (Huang et al., 2015).
The incorporation of silver into wound dressings not only provides antimicrobial protection but also promotes a moist wound environment, which is critical for effective healing. Studies have shown that silver dressings can reduce inflammation and enhance the proliferation of fibroblasts and keratinocytes, essential cells for tissue regeneration (Pavlík et al., 2021; Safaee-Ardakani et al., 2022).
The efficacy of silver ions arises from several key mechanisms:
- Cell Membrane Disruption: Silver ions can penetrate bacterial cell walls and interact with the phospholipid bilayer of the cytoplasmic membrane. This interaction disrupts membrane integrity, leading to increased permeability and eventual cell lysis (Ricco, 2006; Castellano et al., 2007). The binding of silver ions to membrane proteins can also inhibit essential cellular functions, contributing to the bactericidal effect (Castellano et al., 2007).
- Inhibition of Cellular Respiration: Silver ions interfere with the respiratory chain of bacteria by binding to thiol groups in proteins, which are crucial for cellular respiration and energy production. This binding disrupts electron transport processes, ultimately leading to a decrease in adenosine triphosphate (ATP) production and cellular energy depletion (Asharani et al., 2008; Sim et al., 2018). The resulting metabolic disruption is particularly effective against aerobic bacteria, which rely heavily on these pathways for survival.
- Generation of Reactive Oxygen Species (ROS): Silver nanoparticles (AgNPs) can induce oxidative stress in microbial cells by generating reactive oxygen species. This oxidative stress damages cellular components, including lipids, proteins, and DNA, leading to cell death (Tang & Zheng, 2018; Paladini, 2019). The ability of AgNPs to produce ROS is enhanced by their high surface area and reactivity, making them particularly effective against biofilm-forming bacteria, which are often resistant to conventional antibiotics (Paladini, 2019).
- Biofilm Disruption: One of the significant challenges in wound care is the presence of biofilms, which are structured communities of bacteria encased in a self-produced extracellular matrix. Silver has been shown to penetrate and disrupt biofilms, allowing for better penetration of other antimicrobial agents and enhancing overall wound healing (Paladini, 2019; Tang & Zheng, 2018). This property is critical in treating chronic wounds, where biofilm formation is a common barrier to healing.
- Anti-inflammatory Effects: Beyond its antimicrobial properties, silver also exhibits anti-inflammatory effects, which can facilitate wound healing. By modulating the inflammatory response, silver can help reduce excessive inflammation that often complicates wound healing processes (Haidari et al., 2020; May et al., 2021). This dual action—combating infection while promoting a favorable healing environment—makes silver a valuable component in wound care formulations.
- Dose-Dependent Efficacy: The effectiveness of silver is dose-dependent, with higher concentrations generally leading to increased antimicrobial activity. However, it is crucial to balance this with the potential for cytotoxicity to human cells, such as keratinocytes and fibroblasts, which are vital for wound healing (Barnea et al., 2009; May et al., 2021). Studies have indicated that while silver can effectively reduce bacterial load, excessive concentrations may delay re-epithelialization and tissue regeneration (Barnea et al., 2009).
- Resistance Mechanisms: Although silver is effective against many pathogens, some bacteria have developed resistance mechanisms. These include the efflux of silver ions and the alteration of cellular targets, which can diminish the efficacy of silver-based treatments (Monneau et al., 2023; Tyavambiza et al., 2022). Understanding these resistance mechanisms is essential for optimizing silver use in clinical settings and developing strategies to mitigate resistance.
Precautions and Contraindications
While silver has demonstrated significant benefits in wound care, clinicians must be aware of potential precautions and contraindications associated with its use. Prolonged exposure to silver can lead to argyria, a condition characterized by a bluish-gray discoloration of the skin due to silver accumulation (Khansa et al., 2019). Therefore, it is crucial to limit the duration of silver dressing applications, particularly in non-infected wounds, to avoid cytotoxic effects on keratinocytes and fibroblasts (Khansa et al., 2019).
In patients with known hypersensitivity to silver or its compounds, the use of silver dressings should be avoided. Additionally, clinicians should consider the patient’s overall health status, including any comorbid conditions that may affect wound healing, such as diabetes or vascular insufficiency (Weller et al., 2020).
Monitoring for signs of systemic absorption is also important, as studies have indicated that silver can be detected in the bloodstream following the use of silver-containing dressings (Pfurtscheller et al., 2014). Regular assessment of the wound and surrounding tissue is essential to ensure that silver dressings are providing the intended benefits without adverse effects.
Conclusion
The use of silver in wound care represents a significant advancement in the management of both acute and chronic wounds. Its historical application, coupled with a robust understanding of its mechanisms of action, underscores the importance of silver as a therapeutic agent in modern medicine. However, clinicians must remain vigilant regarding the potential risks associated with silver use, ensuring that treatment is tailored to the individual patient’s needs and circumstances.
References:
- Asharani, P., Mun, G., Hande, M., & Valiyaveettil, S. (2008). Cytotoxicity and genotoxicity of silver nanoparticles in human cells. Acs Nano, 3(2), 279-290. https://doi.org/10.1021/nn800596w
- Barnea, Y., Weiss, J., & Gur, E. (2009). A review of the applications of the hydrofiber dressing with silver (aquacel ag®) in wound care. Therapeutics and Clinical Risk Management, 21. https://doi.org/10.2147/tcrm.s3462
- Castellano, J., Shafii, S., Ko, F., Donate, G., Wright, T., Mannari, R., … & Robson, M. (2007). Comparative evaluation of silver‐containing antimicrobial dressings and drugs. International Wound Journal, 4(2), 114-122. https://doi.org/10.1111/j.1742-481x.2007.00316.x
- Chuangsuwanich, A., Chortrakarnkij, P., & Kangwanpoom, J. (2013). Cost-effectiveness analysis in comparing alginate silver dressing with silver zinc sulfadiazine cream in the treatment of pressure ulcers. Archives of Plastic Surgery, 40(05), 589-596. https://doi.org/10.5999/aps.2013.40.5.589
- Dakal, T., Kumar, A., Majumdar, R., & Yadav, V. (2016). Mechanistic basis of antimicrobial actions of silver nanoparticles. Frontiers in Microbiology, 7. https://doi.org/10.3389/fmicb.2016.01831
- Domínguez, A., Ayerbe-Algaba, R., Miró-Canturri, A., Rodríguez-Villodres, Á., & Smani, Y. (2020). Antibacterial activity of colloidal silver against gram-negative and gram-positive bacteria. Antibiotics, 9(1), 36. https://doi.org/10.3390/antibiotics9010036
- Haidari, H., Garg, S., Vasilev, K., Kopecki, Z., & Cowin, A. (2020). Silver-based wound dressings: current issues and future developments for treating bacterial infections. Wound Practice and Research, 28(4). https://doi.org/10.33235/wpr.28.4.173-180
- Huang, X., Liu, Y., Chang, C., Jiao, L., Hang, R., & Tang, B. (2015). A self-regulating antimicrobial model based on the ion-exchange stimuli. Journal of Materials Science Materials in Medicine, 26(7). https://doi.org/10.1007/s10856-015-5546-8
- Khansa, I., Schoenbrunner, A., Kraft, C., & Janis, J. (2019). Silver in wound care—friend or foe?: a comprehensive review. Plastic and Reconstructive Surgery Global Open, 7(8), e2390. https://doi.org/10.1097/gox.0000000000002390
- May, A., Kopecki, Z., Carney, B., & Cowin, A. (2021). Antimicrobial silver dressings: a review of emerging issues for modern wound care. Anz Journal of Surgery, 92(3), 379-384. https://doi.org/10.1111/ans.17382
- Mei, L., Lu, Z., Zhang, X., Li, C., & Jia, Y. (2014). Polymer-ag nanocomposites with enhanced antimicrobial activity against bacterial infection. Acs Applied Materials & Interfaces, 6(18), 15813-15821. https://doi.org/10.1021/am502886m
- Monneau, Y., Arrault, C., Duroux, C., Martin, M., Chirot, F., MacAleese, L., … & Hologne, M. (2023). Structural and dynamical insights into sile silver binding from combined analytical probes. Physical Chemistry Chemical Physics, 25(4), 3061-3071. https://doi.org/10.1039/d2cp04206a
- Nqakala, Z., Sibuyi, N., Fadaka, A., Meyer, M., Onani, M., & Madiehe, A. (2021). Advances in nanotechnology towards development of silver nanoparticle-based wound-healing agents. International Journal of Molecular Sciences, 22(20), 11272. https://doi.org/10.3390/ijms222011272
- Paladini, F. (2019). Antimicrobial silver nanoparticles for wound healing application: progress and future trends. Materials, 12(16), 2540. https://doi.org/10.3390/ma12162540
- Pavlík, V., Sobótka, L., Pejchal, J., Čepa, M., Nešporová, K., Arenbergerová, M., … & Velebný, V. (2021). Silver distribution in chronic wounds and the healing dynamics of chronic wounds treated with dressings containing silver and octenidine. The Faseb Journal, 35(5). https://doi.org/10.1096/fj.202100065r
- Pfurtscheller, K., Petnehazy, T., Goessler, W., Bubalo, V., Kamolz, L., & Trop, M. (2014). Transdermal uptake and organ distribution of silver from two different wound dressings in rats after a burn trauma. Wound Repair and Regeneration, 22(5), 654-659. https://doi.org/10.1111/wrr.12209
- Ricco, J. (2006). Intergard silver bifurcated graft: features and results of a multicenter clinical study. Journal of Vascular Surgery, 44(2), 339-346. https://doi.org/10.1016/j.jvs.2006.03.046
- Safaee-Ardakani, M., Hatamian-Zarmi, A., Sadat, S., Alvandi, H., Ebrahimi-Hosseinzadeh, B., Mokhtari-Hosseini, Z., … & Mohammadi, M. (2022). In vivo study of beta-glucan-based biogenic synthesis of silver nanocomposite using schizophyllum commune for wound dressings in a rat burn model. Advances in Natural Sciences Nanoscience and Nanotechnology, 13(3), 035001. https://doi.org/10.1088/2043-6262/ac79b6
- Sim, W., Barnard, R., Blaskovich, M., & Ziora, Z. (2018). Antimicrobial silver in medicinal and consumer applications: a patent review of the past decade (2007–2017). Antibiotics, 7(4), 93. https://doi.org/10.3390/antibiotics7040093
- Sim, W., Barnard, R., Blaskovich, M., & Ziora, Z. (2018). Antimicrobial silver in medicinal and consumer applications: a patent review of the past decade (2007–2017). Antibiotics, 7(4), 93. https://doi.org/10.3390/antibiotics7040093
- Tang, S. and Zheng, J. (2018). Antibacterial activity of silver nanoparticles: structural effects. Advanced Healthcare Materials, 7(13). https://doi.org/10.1002/adhm.201701503
- Tsaia, C., Hsua, H., & Lina, C. (2014). Treatment of chronic wounds with the silver-containing activated carbon fiber dressing: three cases. Journal of Medical Cases, 5(11), 587-591. https://doi.org/10.14740/jmc1960w
- Tyavambiza, C., Meyer, M., & Meyer, S. (2022). Cellular and molecular events of wound healing and the potential of silver based nanoformulations as wound healing agents. Bioengineering, 9(11), 712. https://doi.org/10.3390/bioengineering9110712
- Weller, C., Team, V., & Sussman, G. (2020). First-line interactive wound dressing update: a comprehensive review of the evidence. Frontiers in Pharmacology, 11. https://doi.org/10.3389/fphar.2020.00155