Associated References
Main Article
The aetiology of wounds and burns in patients undergoing radiotherapy is multifaceted. Radiotherapy can induce skin damage, leading to acute and chronic wounds. The primary mechanism involves ionizing radiation causing direct DNA damage to the skin cells, particularly affecting rapidly dividing cells such as keratinocytes and fibroblasts (Deptuła et al., 2019). This damage can result in impaired wound healing, increased fragility, and susceptibility to infections (Abouarab et al., 2017). Additionally, factors such as the total radiation dose, the fractionation schedule, and the area of the body being treated significantly influence the severity of skin reactions (Fallah et al., 2020).
Moreover, the presence of comorbidities, such as diabetes or vascular diseases, can exacerbate the wound healing process, making patients more vulnerable to complications (Deptuła et al., 2019). The interaction between radiotherapy and other treatments, such as chemotherapy, can further complicate the aetiology of wounds, as these therapies can also impair the skin’s regenerative abilities (Deptuła et al., 2019).
Prevalence of Wounds and Burns in radiotherapy patients
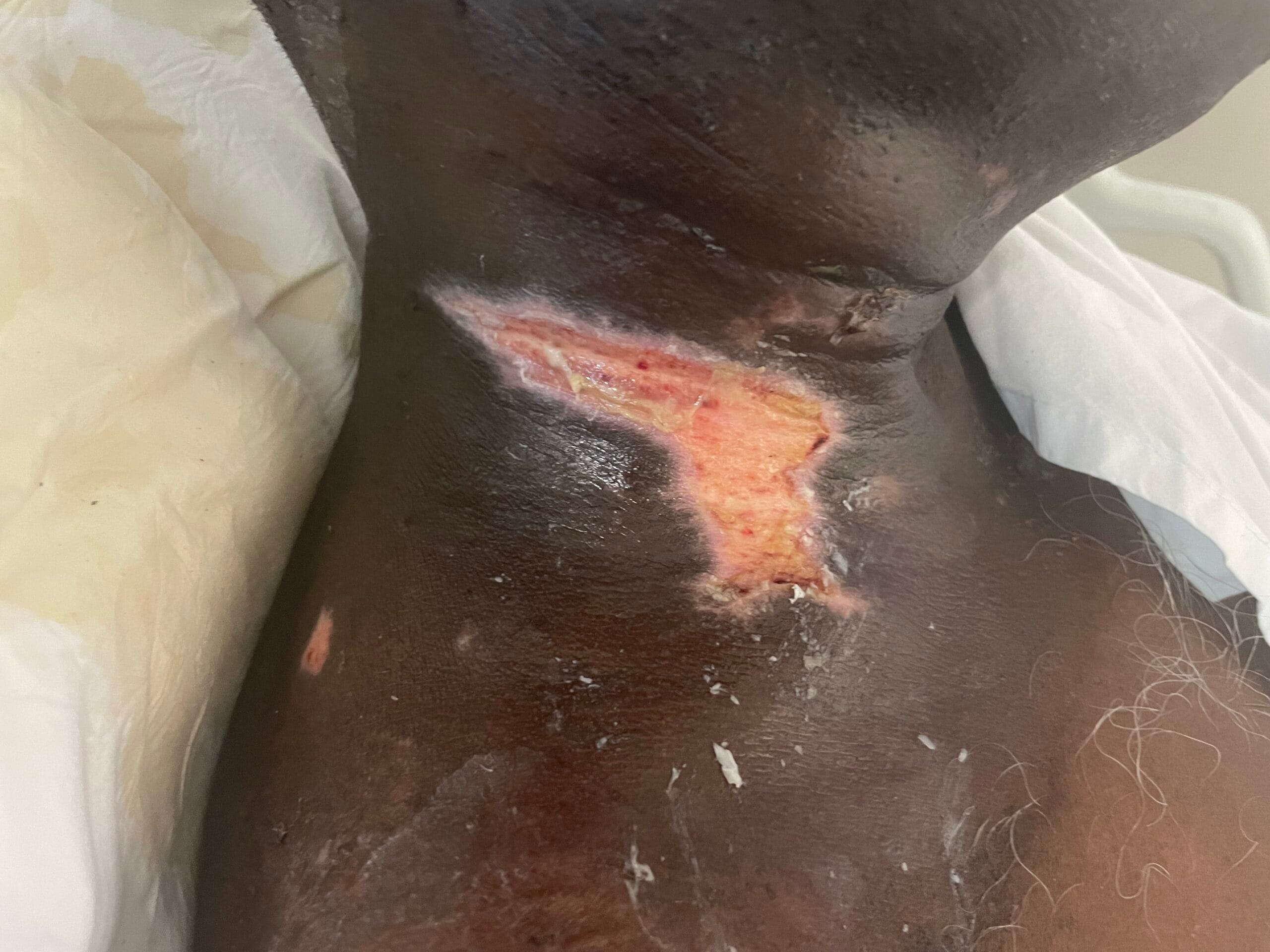
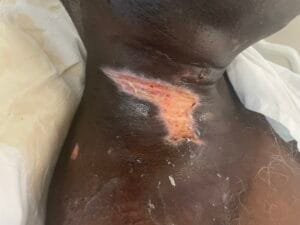
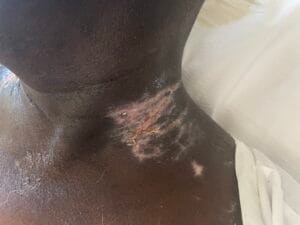
Recent studies indicate that the prevalence of radiotherapy-induced wounds and burns is significant, with estimates suggesting that up to 95% of patients receiving radiotherapy experience some form of skin toxicity, ranging from mild erythema to severe dermatitis (Dréno et al., 2021). In a systematic review, it was reported that the incidence of severe skin reactions varies based on the treatment area, with head and neck cancer patients being particularly susceptible due to the high radiation doses typically administered in these regions (Dréno et al., 2021).
Furthermore, a study highlighted that approximately 100 million people in developed countries acquire scars from surgical procedures annually, with a considerable portion of these being related to oncological treatments, including radiotherapy (Dréno et al., 2021). This underscores the importance of effective wound management strategies in clinical practice.
Pathophysiological Changes
The pathophysiological changes associated with radiotherapy-induced wounds involve a complex interplay of cellular and molecular responses. Ionizing radiation leads to the generation of reactive oxygen species (ROS), which can cause oxidative stress and inflammation, further damaging the surrounding tissues (Fallah et al., 2020). The dysregulation of cytokines and growth factors, such as interleukin-6 (IL-6) and transforming growth factor-beta (TGF-β), plays a critical role in the inflammatory response and subsequent wound healing process (Fallah et al., 2020).
Additionally, radiation can disrupt the extracellular matrix (ECM) composition, impairing fibroblast function and collagen deposition, which are essential for proper wound healing (Deptuła et al., 2019). Studies have shown that patients who undergo preoperative radiotherapy are at a higher risk of postoperative wound complications due to these pathophysiological changes (Haubner et al., 2015).
Treatments for Radiotherapy-Induced Wounds
Current evidence-based treatment options for radiotherapy-induced wounds include various modalities aimed at enhancing healing and preventing complications. Advanced wound dressings, such as hydrocolloids and alginates, have been shown to provide a moist environment conducive to healing while protecting the wound from external contaminants (Thomas et al., 2015). Furthermore, the application of growth factors and stem cell therapies is gaining traction, with studies indicating that adipose-derived stem cells can significantly enhance the healing of irradiated wounds (Mashiko et al., 2018).
Additionally, the use of recombinant human collagen peptides has demonstrated efficacy in improving wound healing outcomes by promoting cellular proliferation and ECM remodeling (Mashiko et al., 2018). The integration of these advanced therapies into clinical practice is essential for optimizing patient outcomes and minimizing the long-term effects of radiotherapy.
Precautions with Treatments
While various treatment options exist, specific precautions and contraindications must be considered. For instance, the use of certain topical agents may exacerbate skin irritation or allergic reactions in sensitive patients (Thomas et al., 2015). Moreover, the timing of interventions, such as the application of radiotherapy in relation to surgical procedures, is crucial; improper timing can lead to increased wound dehiscence and complications (Abouarab et al., 2017).
Clinicians must also be aware of the potential for systemic effects of radiotherapy on immune function, which can further complicate wound healing (Yazdi et al., 2016). Therefore, a multidisciplinary approach involving oncologists, dermatologists, and wound care specialists is vital to ensure comprehensive management of radiotherapy-induced wounds.
Diagnostic Tests Available
Diagnostic methods for confirming wounds and burns related to radiotherapy include clinical assessments, imaging studies, and laboratory tests. Clinicians typically perform a thorough physical examination to assess the extent and severity of the wounds (Thomas et al., 2015). Imaging techniques, such as ultrasound or MRI, can be employed to evaluate deeper tissue involvement and rule out complications such as abscess formation (Thomas et al., 2015).
Laboratory tests, including cultures of wound exudate, can help identify infections that may complicate the healing process (Thomas et al., 2015). The integration of these diagnostic modalities is essential for developing an effective treatment plan tailored to the individual patient’s needs.
Contributing Factors
Understanding the contributing factors to radiotherapy-induced wounds is critical for prevention and management. Modifiable factors include nutritional status, which can significantly influence wound healing; malnutrition can impair immune function and delay recovery (Nugent et al., 2013). Additionally, patient education on skin care and hygiene practices can reduce the risk of infection and promote healing (Thomas et al., 2015).
Non-modifiable factors, such as age and genetic predisposition, also play a role in wound healing outcomes. Older patients often exhibit slower healing rates due to age-related changes in skin structure and function (Deptuła et al., 2019). Furthermore, the presence of comorbidities, such as diabetes or vascular disorders, can complicate the healing process and increase the risk of chronic wounds (Deptuła et al., 2019).
Conclusion
In summary, the management of radiotherapy-induced wounds and burns requires a comprehensive understanding of their aetiology, prevalence, pathophysiological changes, treatment options, precautions, diagnostic methods, and contributing factors. By synthesizing the latest evidence-based practices and fostering a multidisciplinary approach, health clinicians can optimize patient outcomes and enhance the quality of care for individuals undergoing radiotherapy.
References:
- Abouarab, M., Salem, I., Degheidy, M., Henn, D., Hirche, C., Eweida, A., … & Kremer, T. (2017). Therapeutic options and postoperative wound complications after extremity soft tissue sarcoma resection and postoperative external beam radiotherapy. International Wound Journal, 15(1), 148-158. https://doi.org/10.1111/iwj.12851
- Deptuła, M., Zieliński, J., Wardowska, A., & Pikuła, M. (2019). Wound healing complications in oncological patients: perspectives for cellular therapy. Advances in Dermatology and Allergology, 36(2), 139-146. https://doi.org/10.5114/ada.2018.72585
- Dréno, B., Amici, J., Demessant, A., Wright, C., Taïeb, C., Desai, S., … & Alexis, A. (2021). The impact of acne, atopic dermatitis, skin toxicities and scars on quality of life and the importance of a holistic treatment approach. Clinical Cosmetic and Investigational Dermatology, Volume 14, 623-632. https://doi.org/10.2147/ccid.s315846
- Fallah, M., Viklund, E., Bäckman, A., Broden, J., Lundskog, B., Johansson, M., … & Ny, T. (2020). Plasminogen is a master regulator and a potential drug candidate for the healing of radiation wounds. Cell Death and Disease, 11(3). https://doi.org/10.1038/s41419-020-2397-0
- Haubner, F., Pohl, F., Schreml, S., Prantl, L., & Gassner, H. (2015). A co-culture model of fibroblasts and adipose tissue-derived stem cells reveals new insights into impaired wound healing after radiotherapy. International Journal of Molecular Sciences, 16(11), 25947-25958. https://doi.org/10.3390/ijms161125935
- Mashiko, T., Takada, H., Wu, S., Kanayama, K., Feng, J., Tashiro, K., … & Yoshimura, K. (2018). Therapeutic effects of a recombinant human collagen peptide bioscaffold with human adipose-derived stem cells on impaired wound healing after radiotherapy. Journal of Tissue Engineering and Regenerative Medicine, 12(5), 1186-1194. https://doi.org/10.1002/term.2647
- Nugent, B., Lewis, S., & O’Sullivan, J. (2013). Enteral feeding methods for nutritional management in patients with head and neck cancers being treated with radiotherapy and/or chemotherapy. Cochrane Database of Systematic Reviews, 2013(1). https://doi.org/10.1002/14651858.cd007904.pub3
- Thomas, S., Reimer-Kirkham, S., & Kohr, R. (2015). Wound dressings during radiotherapy for cancer: a survey of practice. Clinical Journal of Oncology Nursing, 19(4), E87-E91. https://doi.org/10.1188/15.cjon.e87-e91
- Yazdi, M., Schinkelshoek, M., Loof, N., Taube, C., Hiemstra, P., Welters, M., … & Burg, S. (2016). Standard radiotherapy but not chemotherapy impairs systemic immunity in non-small cell lung cancer. Oncoimmunology, 5(12), e1255393. https://doi.org/10.1080/2162402x.2016.1255393