Associated References
Associated Care Plans/Tutorials
Main Article
Aetiology
Pyoderma gangrenosum (PG) is a rare inflammatory skin disorder characterized by painful ulcerations, often associated with systemic diseases, particularly those involving immune dysregulation. The precise aetiology of PG remains elusive, but it is recognized as a neutrophilic dermatosis, suggesting a significant role of neutrophils in its pathogenesis. Genetic predispositions, particularly mutations in the PSTPIP1 gene, have been implicated in certain autoinflammatory syndromes associated with PG, such as PAPA (pyogenic arthritis, pyoderma gangrenosum, and acne) and PASH (pyoderma gangrenosum, acne, and suppurative hidradenitis) syndromes (Starnes et al., 2014; Cugno et al., 2017; Marzano et al., 2016). Environmental triggers, including infections, trauma, and surgical procedures, can precipitate the onset of PG lesions, indicating that both intrinsic and extrinsic factors contribute to its development (Fletcher et al., 2019; Gameiro et al., 2015; Chokoeva et al., 2016).
The dysregulation of the immune system, particularly involving the innate immune response, is central to the pathophysiology of PG. Studies have shown that patients with PG often exhibit elevated levels of pro-inflammatory cytokines, such as interleukin-1 and tumor necrosis factor-alpha, which are critical mediators of inflammation (Cugno et al., 2017; Chokoeva et al., 2016). Furthermore, the association of PG with inflammatory bowel diseases (IBD) underscores the importance of systemic inflammation in its aetiology, as PG is more prevalent in patients with ulcerative colitis compared to Crohn’s disease (Yang et al., 2018; Jang et al., 2019). The complex interplay between genetic factors, immune dysregulation, and environmental triggers necessitates a comprehensive understanding of PG’s aetiology for effective management.
Prevalence
The prevalence of pyoderma gangrenosum varies across different populations and underlying conditions. Recent studies indicate that PG occurs in approximately 1% to 10% of patients with ulcerative colitis and 0.5% to 20% of those with Crohn’s disease (Yang et al., 2018; Jang et al., 2019). Overall, PG is considered rare, affecting about 3 to 10 individuals per million people annually (Hrin et al., 2021). The condition is more frequently diagnosed in adults, particularly between the ages of 20 and 50, with a slight female predominance noted in many studies (Ohtsuka & Yamamoto, 2014; Alavi et al., 2017).
Geographical variations in the prevalence of PG have also been observed, with certain populations, such as those in Latin America, reporting distinct clinical presentations and associations with other diseases (Rodríguez-Zúñiga et al., 2019). The rarity of PG, combined with its association with serious underlying conditions, highlights the need for heightened awareness among healthcare professionals to ensure timely diagnosis and intervention (Duchnowska et al., 2014; Fayyaz, 2018).
Pathophysiological Changes
The pathophysiology of pyoderma gangrenosum is complex and not fully understood, but it is characterized by a dysregulated immune response, particularly involving neutrophils. The condition is classified as a neutrophilic dermatosis, which suggests that neutrophil dysfunction plays a pivotal role in its development (Borda et al., 2019). Histopathological examination of PG lesions typically reveals a dense infiltrate of neutrophils, often accompanied by a mixed inflammatory response, including lymphocytes and plasma cells (Fletcher et al., 2019; Gameiro et al., 2015).
Recent research has identified several key mechanisms that contribute to the pathophysiology of PG, including the activation of the inflammasome pathway, which leads to the production of pro-inflammatory cytokines (Chokoeva et al., 2016; Marzano et al., 2016). Additionally, genetic factors, such as mutations in the PSTPIP1 gene, have been linked to the development of PG in certain autoinflammatory syndromes, further elucidating the genetic basis of the disease (Starnes et al., 2014; Cugno et al., 2017). The interplay between innate and adaptive immune responses, along with environmental triggers, underscores the multifactorial nature of PG’s pathophysiology, necessitating a comprehensive approach to treatment and management.
Treatments
Current evidence-based treatment options for pyoderma gangrenosum include both pharmacological and non-pharmacological interventions. First-line treatments typically involve systemic corticosteroids, which are effective in reducing inflammation and promoting healing of ulcerative lesions (Fletcher et al., 2019; Alavi et al., 2017). In cases where corticosteroids are insufficient or contraindicated, immunosuppressive agents such as cyclosporine, mycophenolate mofetil, and biologics like infliximab have shown promise in managing PG (Hrin et al., 2021; Crouse et al., 2018).
Non-pharmacological interventions, including wound care and surgical options, may also play a role in the management of PG. Proper wound care is essential to prevent secondary infections and promote healing, while surgical debridement may be necessary in cases of extensive tissue necrosis (Schotanus et al., 2014). Emerging therapies targeting specific inflammatory pathways, such as interleukin-1 inhibitors, are currently under investigation and may offer new avenues for treatment in the future (Chokoeva et al., 2016; Marzano et al., 2016).
Precautions with Treatments
While various treatment options are available for pyoderma gangrenosum, clinicians must be aware of specific precautions and contraindications associated with these therapies. Systemic corticosteroids, while effective, can lead to significant side effects, including increased susceptibility to infections, osteoporosis, and metabolic disturbances (Fletcher et al., 2019; Alavi et al., 2017). Immunosuppressive agents, such as cyclosporine and mycophenolate mofetil, also carry risks of infection and require careful monitoring of renal function and blood counts (Hrin et al., 2021; Crouse et al., 2018).
Biologic therapies, although generally well-tolerated, may also pose risks, including infusion reactions and the potential for reactivation of latent infections, such as tuberculosis (Fletcher et al., 2019; Gameiro et al., 2015). Therefore, a thorough assessment of the patient’s medical history and comorbid conditions is essential before initiating treatment. Additionally, clinicians should educate patients about the signs of infection and the importance of adhering to follow-up appointments to monitor treatment efficacy and adverse effects (Fayyaz, 2018; (Fletcher et al., 2019; .
Diagnostic Tests Available
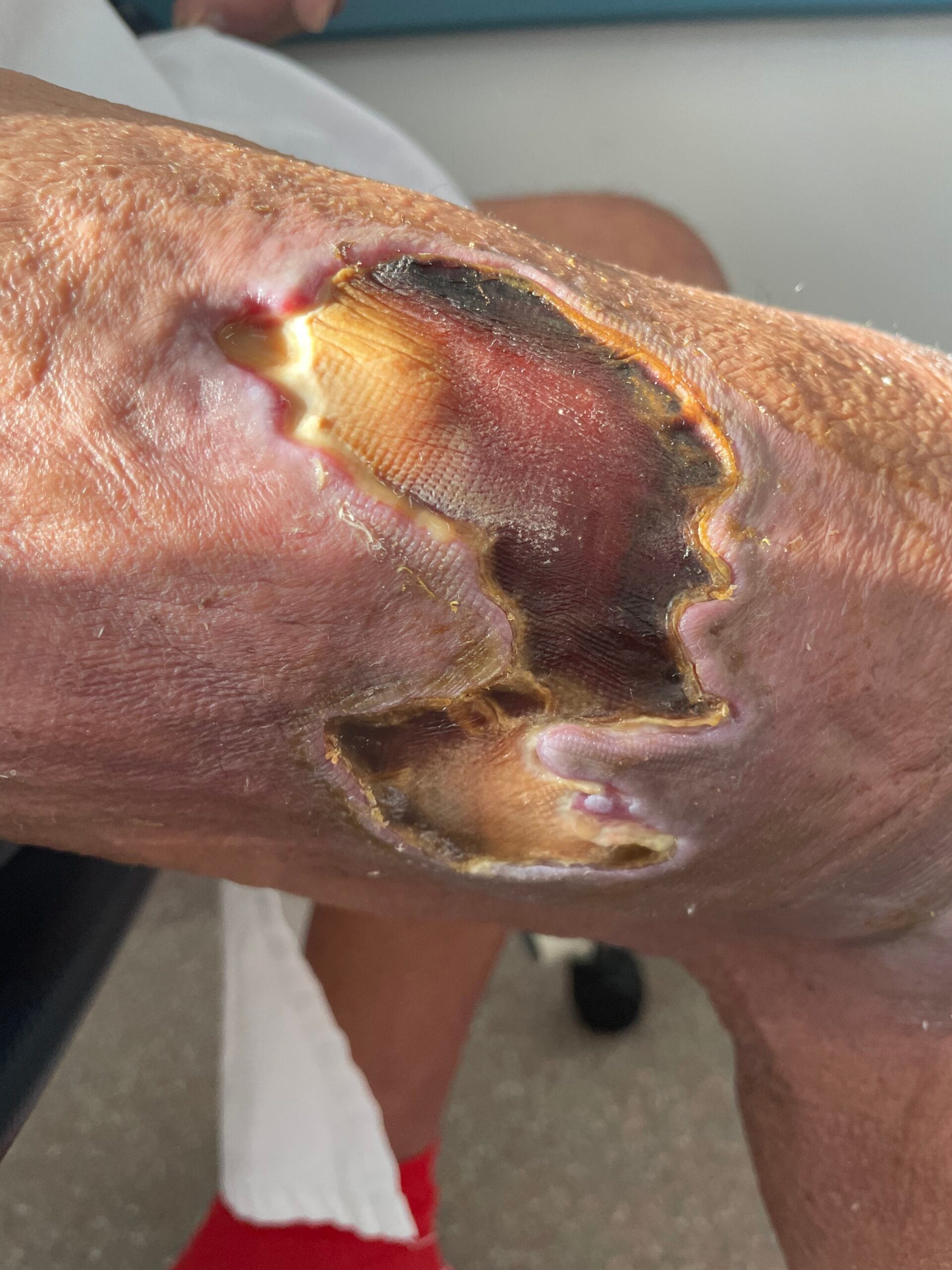
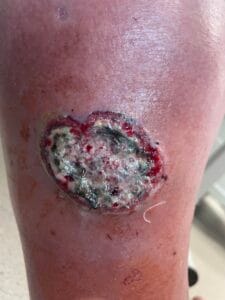
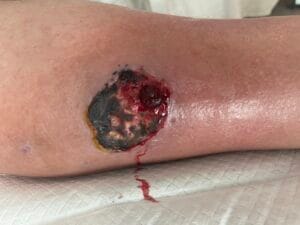
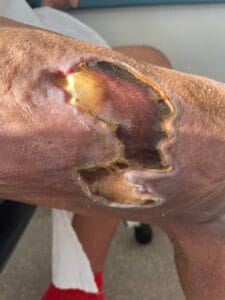
Diagnosing pyoderma gangrenosum can be challenging due to its clinical similarity to other ulcerative skin conditions. Currently, there are no specific laboratory tests or histopathological findings that definitively confirm PG; thus, diagnosis is often based on clinical criteria and exclusion of other conditions (Haag et al., 2021). The most widely accepted diagnostic criteria include the presence of painful, rapidly progressing ulcers with undermined borders, often accompanied by systemic symptoms (Haag et al., 2021; Fletcher et al., 2019).
A thorough clinical evaluation, including a detailed patient history and physical examination, is crucial for accurate diagnosis. In some cases, skin biopsy may be performed to rule out other conditions, such as infections or malignancies, although the biopsy findings are typically non-specific (Schotanus et al., 2014; Gameiro et al., 2015). Additionally, laboratory tests to assess for underlying systemic diseases, such as inflammatory bowel disease or autoimmune disorders, may be warranted to guide treatment decisions (Duchnowska et al., 2014; Yang et al., 2018; Fletcher et al., 2019).
Contributing Factors
Several factors may predispose individuals to develop pyoderma gangrenosum, including comorbid conditions and genetic predispositions. The condition is frequently associated with systemic diseases, particularly inflammatory bowel diseases, rheumatoid arthritis, and certain malignancies (Yang et al., 2018; Borda et al., 2019; Fletcher et al., 2019). Patients with a history of autoimmune disorders or autoinflammatory syndromes, such as PAPA and PASH, are also at increased risk for developing PG (Cugno et al., 2017; Marzano et al., 2016).
Environmental factors, including trauma, surgery, and infections, can act as triggers for the onset of PG lesions in susceptible individuals (Starnes et al., 2014; Fletcher et al., 2019). Furthermore, the presence of chronic inflammation, whether due to underlying disease or external factors, may exacerbate the condition and complicate management (Borda et al., 2019; (Gameiro et al., 2015; . Understanding these contributing factors is essential for clinicians to identify at-risk patients and implement appropriate preventive measures.
Conclusion
In conclusion, pyoderma gangrenosum is a complex and multifaceted condition that poses significant challenges for diagnosis and management. A thorough understanding of its aetiology, prevalence, pathophysiological changes, treatment options, and contributing factors is essential for healthcare professionals involved in the care of affected patients. Ongoing research into the underlying mechanisms of PG and the development of targeted therapies will be crucial in improving outcomes for individuals suffering from this debilitating condition.
References:
- Alavi, A., French, L., Davis, M., Brassard, A., & Kirsner, R. (2017). Pyoderma gangrenosum: an update on pathophysiology, diagnosis and treatment. American Journal of Clinical Dermatology, 18(3), 355-372. https://doi.org/10.1007/s40257-017-0251-7
- Borda, L., Wong, L., Marzano, A., & Ortega‐Loayza, A. (2019). Extracutaneous involvement of pyoderma gangrenosum. Archives of Dermatological Research, 311(6), 425-434. https://doi.org/10.1007/s00403-019-01912-1
- Chokoeva, A., Cardoso, J., Wollina, U., & Tchernev, G. (2016). Pyoderma gangrenosum—a novel approach?. Wiener Medizinische Wochenschrift, 167(3-4), 58-65. https://doi.org/10.1007/s10354-016-0472-z
- Crouse, L., McShane, D., Morrell, D., & Wu, E. (2018). Pyoderma gangrenosum in an infant: a case report and review of the literature. Pediatric Dermatology, 35(5). https://doi.org/10.1111/pde.13471
- Cugno, M., Borghi, A., & Marzano, A. (2017). Papa, pash and papash syndromes: pathophysiology, presentation and treatment. American Journal of Clinical Dermatology, 18(4), 555-562. https://doi.org/10.1007/s40257-017-0265-1
- Duchnowska, R., Ziajka, E., Góralska, A., & Grala, B. (2014). Recurrent pyoderma gangrenosum precipitated by breast cancer: a case report and review of the literature. Journal of Medical Case Reports, 8(1). https://doi.org/10.1186/1752-1947-8-226
- Fayyaz, B. (2018). Pyoderma gangrenosum in primary care setting: the challenges involved. Journal of Community Hospital Internal Medicine Perspectives, 8(2), 57-59. https://doi.org/10.1080/20009666.2018.1452518
- Fletcher, J., Alhusayen, R., & Alavi, A. (2019). Recent advances in managing and understanding pyoderma gangrenosum. F1000research, 8, 2092. https://doi.org/10.12688/f1000research.19909.1
- Gameiro, A., Pereira, N., Cardoso, J., & Gonçalo, M. (2015). Pyoderma gangrenosum: challenges and solutions. Clinical Cosmetic and Investigational Dermatology, 285. https://doi.org/10.2147/ccid.s61202
- Haag, C., Hansen, T., Hajar, T., Latour, E., Keller, J., Shinkai, K., … & Ortega‐Loayza, A. (2021). Comparison of three diagnostic frameworks for pyoderma gangrenosum. Journal of Investigative Dermatology, 141(1), 59-63. https://9doi.org/10.1016/j.jid.2020.04.01
- Hrin, M., Bashyam, A., Huang, W., & Feldman, S. (2021). Mycophenolate mofetil as adjunctive therapy to corticosteroids for the treatment of pyoderma gangrenosum: a case series and literature review. International Journal of Dermatology, 60(12). https://doi.org/10.1111/ijd.15539
- Jang, H., Kang, B., & Choe, B. (2019). The difference in extraintestinal manifestations of inflammatory bowel disease for children and adults. Translational Pediatrics, 8(1), 4-15. https://doi.org/10.21037/tp.2019.01.06
- Marzano, A., Borghi, A., Meroni, P., & Cugno, M. (2016). Pyoderma gangrenosum and its syndromic forms: evidence for a link with autoinflammation. British Journal of Dermatology, 175(5), 882-891. https://doi.org/10.1111/bjd.14691
- Ohtsuka, M. and Yamamoto, T. (2014). Rare association of pyoderma gangrenosum and palmoplantar pustulosis: a case report and review of the previous works. The Journal of Dermatology, 41(8), 732-735. https://doi.org/10.1111/1346-8138.12543
- Rodríguez-Zúñiga, M., Heath, M., Gontijo, J., & Ortega‐Loayza, A. (2019). Pyoderma gangrenosum: a review with special emphasis on latin america literature. Anais Brasileiros De Dermatologia, 94(6), 729-743. https://doi.org/10.1016/j.abd.2019.06.001
- Schotanus, M., Hout, N., & Vos, D. (2014). Pyoderma gangrenosum of the hand. Advances in Skin & Wound Care, 27(2), 61-64. https://doi.org/10.1097/01.asw.0000441101.33820.e3
- Starnes, T., Bennin, D., Bing, X., Eickhoff, J., Grahf, D., Bellak, J., … & Huttenlocher, A. (2014). The f-bar protein pstpip1 controls extracellular matrix degradation and filopodia formation in macrophages. Blood, 123(17), 2703-2714. https://doi.org/10.1182/blood-2013-07-516948
- Yang, B., Choi, N., Kim, M., Chun, J., Joo, S., Kim, H., … & Lee, J. (2018). Prevalence of extraintestinal manifestations in korean inflammatory bowel disease patients. Plos One, 13(7), e0200363. https://doi.org/10.1371/journal.pone.0200363