Quick Overview
Associated References
Main Article
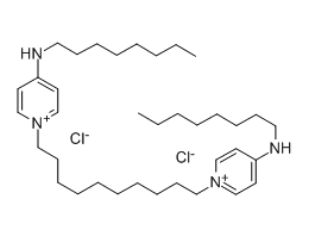
Octenidine dihydrochloride (OCT) is a cationic antiseptic agent that has gained prominence in wound care since its development in the 1980s. Initially, it was introduced as an alternative to traditional antiseptics like chlorhexidine and povidone-iodine, which have been widely used for their antimicrobial properties but also exhibit significant cytotoxicity and adverse effects on wound healing (Assadian, 2016). Over the years, OCT has been recognized for its broad-spectrum antimicrobial activity against both Gram-positive and Gram-negative bacteria, fungi, and some viruses, making it a versatile agent in various clinical settings, including surgical and burn wound care (Joon et al., 2020; Coaguila‐Llerena et al., 2020).
Clinical studies have demonstrated OCT’s efficacy in reducing microbial load in wounds and preventing infections, which is crucial for optimal healing outcomes (Gulzar et al., 2020). Its introduction into clinical practice has been supported by numerous in vitro and animal studies that confirm its safety and tolerability, establishing it as a reliable antiseptic in wound management (Amrita, 2024). The increasing body of evidence has led to its adoption in various formulations, including gels and solutions, specifically designed for wound care applications (Seiser et al., 2021).
Mechanism of Action
The antimicrobial efficacy of octenidine dihydrochloride is primarily attributed to its unique chemical structure, which consists of two cationic active centers that interact with negatively charged components of microbial cell membranes (Joon et al., 2020; Grecka & Szweda, 2021). This interaction disrupts the integrity of the cell membrane, leading to leakage of cytoplasmic contents and ultimately cell lysis (Paleczny et al., 2021). The mechanism is effective against a wide range of pathogens, including methicillin-resistant Staphylococcus aureus (MRSA), which poses a significant challenge in wound management (Kwiatkowski et al., 2019).
OCT’s mode of action is multifaceted. Firstly, its cationic nature allows it to bind effectively to the negatively charged surfaces of bacterial membranes, leading to membrane destabilization and subsequent cell death (Scharf, 2023). This binding is not only rapid but also persistent, allowing OCT to maintain its antimicrobial activity over time, which is particularly beneficial in the context of chronic wounds where bacterial colonization can impede healing (Marco et al., 2022).
Moreover, recent studies have highlighted OCT’s anti-inflammatory properties, which contribute to its overall effectiveness in wound healing. For instance, OCT has been shown to inhibit proteolytic enzymes that can degrade extracellular matrix components, thereby preserving the structural integrity of the wound bed and promoting healing (Cai et al., 2023; Seiser et al., 2021). This protease-inhibitory action is crucial in managing chronic wounds, where excessive protease activity often leads to delayed healing (Seiser et al., 2021).
Additionally, OCT’s ability to form stable complexes with cellular components enhances its tissue compatibility, reducing cytotoxic effects while maintaining its antiseptic efficacy (Vanscheidt et al., 2011). This characteristic is particularly important in wound care, as it allows for effective microbial control without adversely affecting the surrounding healthy tissue (Miller et al., 2010).
Furthermore, OCT exhibits a high residual effect, allowing for prolonged antimicrobial action even after application (Scharf, 2023). This characteristic is particularly beneficial in wound care, where continuous protection against microbial colonization is essential for healing. Studies have indicated that OCT can significantly reduce bioburden in infected wounds, leading to improved healing outcomes compared to traditional antiseptics (Hämmerle & Strohal, 2014).
In summary, the mode of action of octenidine dihydrochloride encompasses membrane disruption, protease inhibition, and enhanced tissue compatibility, all of which contribute to its effectiveness as an antiseptic in wound care. This multifaceted approach not only targets microbial pathogens but also supports the physiological processes necessary for optimal wound healing.
Clinical Precautions and Contraindications
While octenidine dihydrochloride is generally well-tolerated, clinicians must be aware of potential adverse effects and contraindications associated with its use. Some studies have reported instances of localized skin reactions, including erythema and edema, particularly in sensitive populations (Marco et al., 2022). Moreover, excessive application or prolonged exposure to OCT can lead to cytotoxic effects on fibroblasts and keratinocytes, which are critical for wound healing (Cai et al., 2023). Therefore, it is essential to adhere to recommended concentrations and application protocols.
OCT should be used cautiously in patients with known hypersensitivity to the compound or its components. Additionally, its use in deep wounds or cavities may necessitate careful consideration, as the temperature-dependent properties of OCT could influence its efficacy and safety profile (Mainka et al., 2020). Clinicians should also be vigilant about potential interactions with other antiseptics or topical agents, as these could alter the effectiveness of OCT or exacerbate adverse effects (Coaguila‐Llerena et al., 2020).
Conclusion
In summary, octenidine dihydrochloride represents a significant advancement in wound care, offering a potent antimicrobial solution with favorable safety and efficacy profiles. Its historical development, coupled with a well-understood mechanism of action, underscores its role as a valuable antiseptic in clinical practice. However, healthcare providers must remain aware of the precautions and potential adverse effects associated with its use to ensure optimal patient outcomes.
References:
- Amrita, A. (2024). Comparative evaluation of octenidine with chlorhexidine mouthwash in gingivitis and periodontitis patients: a randomized clinical trial. Journal of Pharmacy and Bioallied Sciences, 16(Suppl 1), S789-S791. https://doi.org/10.4103/jpbs.jpbs_1011_23
- Assadian, O. (2016). Octenidine dihydrochloride: chemical characteristics and antimicrobial properties. Journal of Wound Care, 25(Sup3), S3-S6. https://doi.org/10.12968/jowc.2016.25.sup3.s3
- Cai, X., Venkatesan, J., Schmitt, G., Reda, B., Cucchiarini, M., Hannig, M., … & Madry, H. (2023). Cytotoxic effects of different mouthwash solutions on primary human articular chondrocytes and normal human articular cartilage – an in vitro study. Clinical Oral Investigations, 27(9), 4987-5000. https://doi.org/10.1007/s00784-023-05118-8
- Coaguila‐Llerena, H., Rodrigues, É., Santos, C., Ramos, S., Medeiros, M., Chávez-Andrade, G., … & Faria, G. (2020). Effects of octenidine applied alone or mixed with sodium hypochlorite on eukaryotic cells. International Endodontic Journal, 53(9), 1264-1274. https://doi.org/10.1111/iej.13347
- Grecka, K. and Szweda, P. (2021). Synergistic effects of propolis combined with 2-phenoxyethanol and antipyretics on the growth of staphylococcus aureus. Pharmaceutics, 13(2), 215. https://doi.org/10.3390/pharmaceutics13020215
- Gulzar, R., Ajitha, P., & Subbaiyan, H. (2020). Comparative evaluation of the antimicrobial efficacy of octenidine dihydrochloride with contemporary root canal disinfectants: a systematic review. Journal of Pharmaceutical Research International, 64-76. https://doi.org/10.9734/jpri/2020/v32i1730669
- Hämmerle, G. and Strohal, R. (2014). Efficacy and cost‐effectiveness of octenidine wound gel in the treatment of chronic venous leg ulcers in comparison to modern wound dressings. International Wound Journal, 13(2), 182-188. https://doi.org/10.1111/iwj.12250
- Joon, A., Khetarpal, A., & Dahiya, S. (2020). Comparitive evaluation of antimicrobial efficacy of 0.1% octenidine dihydrochloride, 2% chlorhexidine and 2% chitosan against e.faecalis within the dentinal tubules. Ip Indian Journal of Conservative and Endodontics, 5(4), 192-199. https://doi.org/10.18231/j.ijce.2020.047
- Kwiatkowski, P., Łopusiewicz, Ł., Kostek, M., Drozłowska, E., Pruss, A., Wojciuk, B., … & Dołęgowska, B. (2019). The antibacterial activity of lavender essential oil alone and in combination with octenidine dihydrochloride against mrsa strains. Molecules, 25(1), 95. https://doi.org/10.3390/molecules25010095
- Mainka, D., Schwarz, J., & Girreser, U. (2020). Temperature dependent analysis of octenidine (n,n´-(decane-1,10-diyldipyridin-1-yl-4-ylidene)dioctan-1-amine) dihydrochloride by nmr and nir spectroscopy. Arkivoc, 2020(6), 287-298. https://doi.org/10.24820/ark.5550190.p011.189
- Marco, O., Hamitaga, F., & Alessia, A. (2022). Subcutaneous tissue swelling and prolonged edema: unexpected outcomes of the disinfection through octenidine dihydrochloride (octenisept®). International Journal of Clinical Pediatrics, 11(1), 9-13. https://doi.org/10.14740/ijcp437
- Miller, C., Newall, N., Kapp, S., Lewin, G., Karimi, L., Carville, K., … & Santamaria, N. (2010). A randomized‐controlled trial comparing cadexomer iodine and nanocrystalline silver on the healing of leg ulcers. Wound Repair and Regeneration, 18(4), 359-367. https://doi.org/10.1111/j.1524-475x.2010.00603.x
- Paleczny, J., Junka, A., Brożyna, M., Dydak, K., Oleksy-Wawrzyniak, M., Ciecholewska-Juśko, D., … & Bartoszewicz, M. (2021). The high impact of staphylococcus aureus biofilm culture medium on in vitro outcomes of antimicrobial activity of wound antiseptics and antibiotic. Pathogens, 10(11), 1385. https://doi.org/10.3390/pathogens10111385
- Reda, B., Dudek, J., Martínez-Hernández, M., & Hannig, M. (2021). Effects of octenidine on the formation and disruption of dental biofilms: an exploratory in situ study in healthy subjects. Journal of Dental Research, 100(9), 950-959. https://doi.org/10.1177/0022034521999044
- Scharf, M. (2023). Preoperative decolonization appears to reduce the risk of infection in high-risk groups undergoing total hip arthroplasty. Antibiotics, 12(5), 877. https://doi.org/10.3390/antibiotics12050877
- Seiser, S., Janker, L., Zila, N., Mildner, M., Rakita, A., Matiasek, J., … & Elbe‐Bürger, A. (2021). Octenidine-based hydrogel shows anti-inflammatory and protease-inhibitory capacities in wounded human skin. Scientific Reports, 11(1). https://doi.org/10.1038/s41598-020-79378-9
- Seiser, S., Janker, L., Zila, N., Mildner, M., Rakita, A., Matiasek, J., … & Elbe‐Bürger, A. (2021). Octenidine-based hydrogel shows anti-inflammatory and protease-inhibitory capacities in wounded human skin. Scientific Reports, 11(1). https://doi.org/10.1038/s41598-020-79378-9
- Vanscheidt, W., Harding, K., Téot, L., & Siebert, J. (2011). Effectiveness and tissue compatibility of a 12‐week treatment of chronic venous leg ulcers with an octenidine based antiseptic – a randomized, double‐blind controlled study. International Wound Journal, 9(3), 316-323. https://doi.org/10.1111/j.1742-481x.2011.00886.x