Quick Overview
Associated References
Main Article
Aetiology
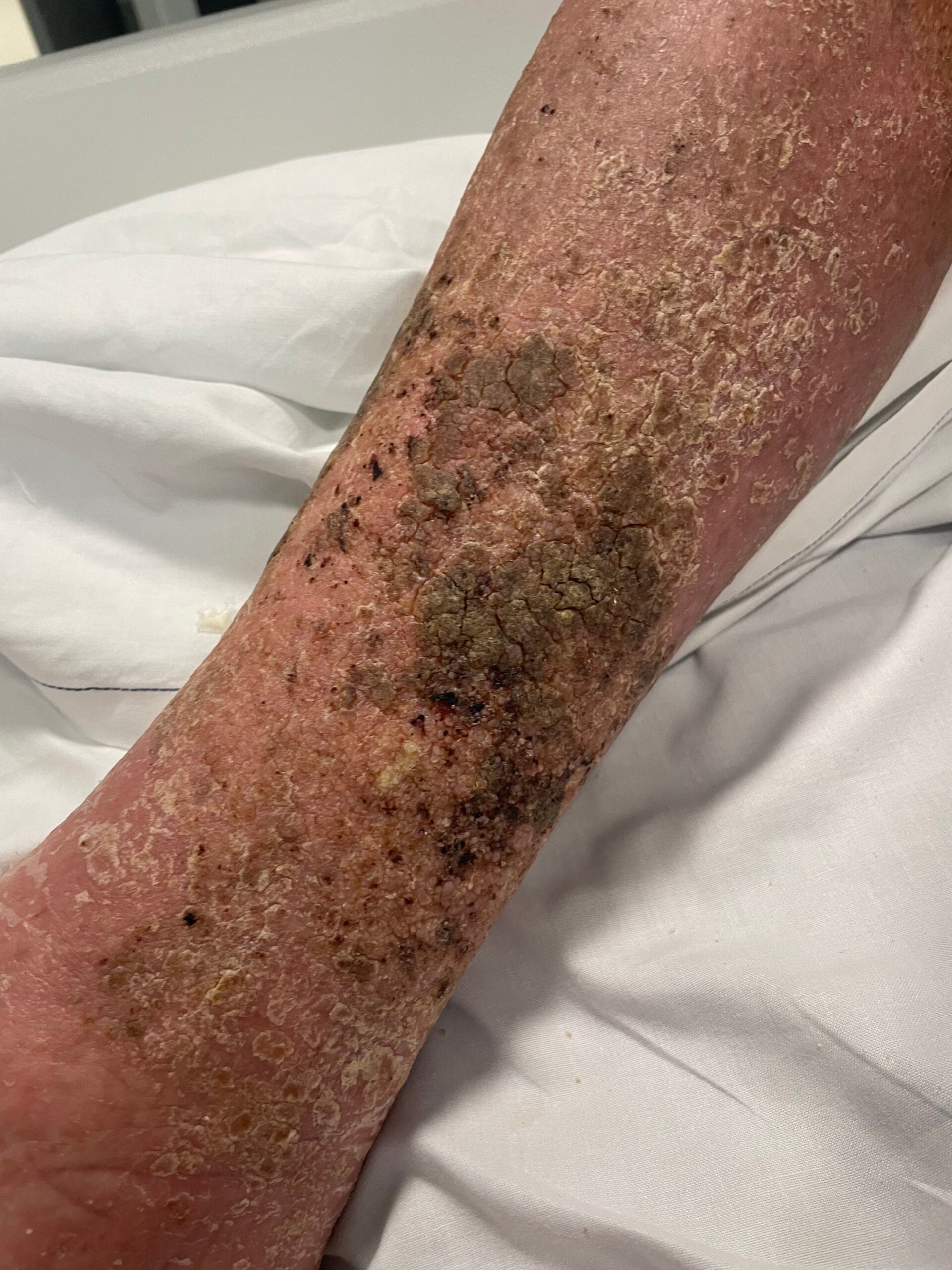
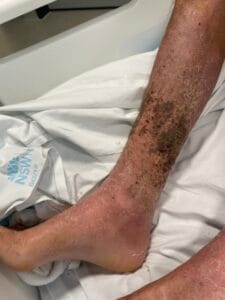
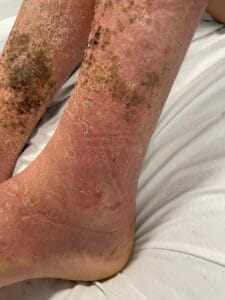
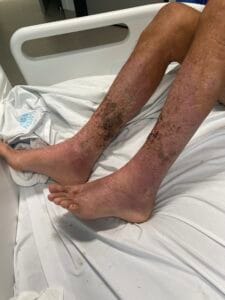
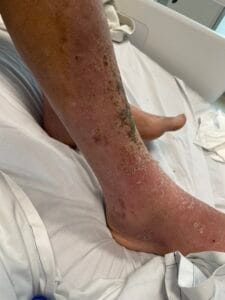
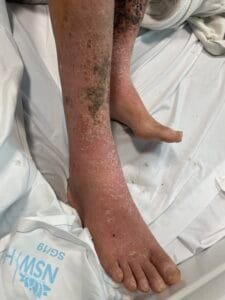
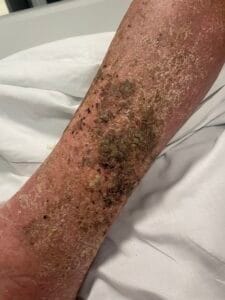
Hyperkeratosis is characterized by the thickening of the stratum corneum, often resulting from various underlying causes. The aetiology of hyperkeratosis can be multifactorial, involving genetic, environmental, and pathological factors. Genetic predispositions play a significant role, particularly in conditions such as ichthyosis and keratosis follicularis spinulosa decalvans, where mutations in specific genes lead to abnormal keratinization processes (Zhang et al., 2016). Environmental factors, including chronic friction or irritation, can also induce hyperkeratosis, as seen in conditions like plantar warts and calluses (Romero-Quintana et al., 2013). Furthermore, inflammatory skin diseases such as psoriasis and eczema can lead to secondary hyperkeratosis due to increased keratinocyte proliferation and altered differentiation (Liu et al., 2014).
Prevalence
The prevalence of hyperkeratosis varies depending on the specific type and underlying condition. For instance, keratosis pilaris, a common form of hyperkeratosis, affects approximately 50-80% of adolescents and young adults (Li et al., 2021). In contrast, conditions like palmoplantar keratoderma, which can also present with hyperkeratosis, have a lower prevalence, estimated at 1-4 cases per million in the general population (Romero-Quintana et al., 2013). Recent studies have highlighted the increasing recognition of hyperkeratosis in various dermatological conditions, emphasizing the need for clinicians to be aware of its manifestations in diverse patient populations (Scalone et al., 2014).
Pathophysiological Changes
The pathophysiological mechanisms underlying hyperkeratosis involve complex interactions between keratinocytes, inflammatory mediators, and environmental stimuli. In conditions like psoriasis, the dysregulation of immune responses leads to increased secretion of cytokines such as IL-17, which promotes keratinocyte proliferation and hyperkeratosis (Liu et al., 2014). Additionally, in oral lichen planus, histopathological features include hyperkeratosis alongside acanthosis and lymphocytic infiltration, indicating an immune-mediated process (Rivera et al., 2020). The interplay between genetic mutations and environmental triggers further complicates the pathophysiology, as seen in ichthyosis, where mutations in transglutaminase genes disrupt normal keratinization (Ortega-Recalde et al., 2015).
Treatments
Current evidence-based treatment options for hyperkeratosis focus on addressing the underlying causes and managing symptoms. Topical therapies, such as keratolytics (e.g., salicylic acid and urea), are commonly used to reduce thickened skin and promote exfoliation (Mazzilli et al., 2018). In cases of inflammatory skin diseases, corticosteroids and immunomodulators like tacrolimus have shown efficacy in reducing inflammation and associated hyperkeratosis (Mazzilli et al., 2018). For more severe or resistant cases, systemic treatments, including retinoids, may be considered, particularly in conditions like psoriasis (Regnault et al., 2015). Recent advances in biologic therapies targeting specific inflammatory pathways have also demonstrated promise in managing hyperkeratosis associated with autoimmune conditions (Liu et al., 2014).
Precautions with Treatments
While treating hyperkeratosis, clinicians must be aware of specific precautions and contraindications associated with various treatment modalities. For instance, topical retinoids can cause skin irritation and should be used cautiously in patients with sensitive skin or those prone to eczema (Regnault et al., 2015). Additionally, systemic retinoids, while effective, carry risks of teratogenicity and require careful monitoring of liver function and lipid levels (Mazzilli et al., 2018). In patients with a history of skin cancer, the use of immunosuppressive agents should be approached with caution due to the potential for increased malignancy risk (DeAngelis et al., 2019). Clinicians should also consider patient-specific factors, such as comorbidities and concurrent medications, when devising treatment plans.
Diagnostic Tests Available
Diagnosing hyperkeratosis typically involves a combination of clinical evaluation and histopathological examination. Clinicians often rely on dermoscopy to assess the characteristics of hyperkeratotic lesions, which can aid in differentiating between various types of keratosis (Iorizzo et al., 2021). Skin biopsies may be warranted in atypical cases to confirm the diagnosis and rule out malignancy, particularly in lesions that exhibit significant changes or are symptomatic (DeAngelis et al., 2019). Additionally, serological tests may be useful in identifying underlying autoimmune conditions that could contribute to hyperkeratosis, such as lichen planus (Rivera et al., 2020).
Contributing Factors
The development of hyperkeratosis is influenced by both modifiable and non-modifiable factors. Non-modifiable factors include genetic predispositions, as seen in hereditary conditions like ichthyosis and keratosis pilaris (Zhang et al., 2016). Modifiable factors encompass environmental influences such as exposure to irritants, friction, and humidity, which can exacerbate or trigger hyperkeratosis (Li et al., 2021). Lifestyle factors, including poor skincare practices and inadequate sun protection, can also contribute to the development of hyperkeratosis, particularly in sun-exposed areas (Scalone et al., 2014). Clinicians should educate patients on the importance of skincare and lifestyle modifications to mitigate the risk of hyperkeratosis.
Conclusion
In summary, hyperkeratosis is a complex dermatological condition with diverse aetiologies, prevalence rates, and pathophysiological mechanisms. Understanding the underlying causes and current treatment options is essential for health clinicians managing this condition. By recognizing the contributing factors and implementing evidence-based interventions, clinicians can improve patient outcomes and enhance the quality of care for individuals affected by hyperkeratosis.
References:
- DeAngelis, L., Cirillo, N., & McCullough, M. (2019). The immunopathogenesis of oral lichen planus—is there a role for mucosal associated invariant t cells?. Journal of Oral Pathology and Medicine, 48(7), 552-559. https://doi.org/10.1111/jop.12898
- Iorizzo, M., Starace, M., Altobrando, A., Alessandrini, A., Veneziano, L., & Piraccini, B. (2021). The value of dermoscopy of the nail plate free edge and hyponychium. Journal of the European Academy of Dermatology and Venereology, 35(12), 2361-2366. https://doi.org/10.1111/jdv.17521
- Li, X., Zhang, J., Zhang, H., & Li, T. (2021). Biopsied non-dental plaque-induced gingival diseases in a chinese population: a single-institute retrospective study. BMC Oral Health, 21(1). https://doi.org/10.1186/s12903-021-01614-z
- Liu, X., Walton, S., Murray, H., King, M., Kelly, A., Holt, D., … & Mounsey, K. (2014). Crusted scabies is associated with increased il‐17 secretion by skin t cells. Parasite Immunology, 36(11), 594-604. https://doi.org/10.1111/pim.12129
- Mazzilli, S., Diluvio, L., Prete, M., Rossi, P., Orlandi, A., Bianchi, L., … & Campione, E. (2018). Tacrolimus 0.03% ointment for treatment of paediatric lichen sclerosus: a case series and literature review. Journal of International Medical Research, 46(9), 3724-3728. https://doi.org/10.1177/0300060518778219
- Ortega-Recalde, O., Moreno, M., Vergara, J., Fonseca, D., Rojas, R., Mosquera, H., … & Laissue, P. (2015). A noveltgm1mutation, leading to multiple splicing rearrangements, is associated with autosomal recessive congenital ichthyosis. Clinical and Experimental Dermatology, 40(7), 757-760. https://doi.org/10.1111/ced.12627
- Regnault, M., Gadaud, N., Boulinguez, S., Tournier, É., Lamant, L., Gladieff, L., … & Sibaud, V. (2015). Chemotherapy-related reticulate hyperpigmentation: a case series and review of the literature. Dermatology, 231(4), 312-318. https://doi.org/10.1159/000439047
- Rivera, C., Crisóstomo, M., Peña, C., González-Díaz, P., & González‐Arriagada, W. (2020). Oral lichen planus interactome reveals cxcr4 and cxcl12 as candidate therapeutic targets. Scientific Reports, 10(1). https://doi.org/10.1038/s41598-020-62258-7
- Romero-Quintana, J., Frías-Castro, L., Arámbula-Meraz, E., Aguilar‐Medina, M., Dueñas-Arias, J., Melchor-Soto, J., … & Ramos‐Payán, R. (2013). Identification of novel mutation in cathepsin c gene causing papillon-lefèvre syndrome in mexican patients. BMC Medical Genetics, 14(1). https://doi.org/10.1186/1471-2350-14-7
- Scalone, L., Cortesi, P., Mantovani, L., Belisari, A., Ayala, F., Fortina, A., … & Giannetti, A. (2014). Clinical epidemiology of hand eczema in patients accessing dermatological reference centres: results from italy. British Journal of Dermatology, 172(1), 187-195. https://doi.org/10.1111/bjd.13220
- Zhang, J., Wang, Y., Cheng, R., Ni, C., Liang, J., Li, M., … & Yao, Z. (2016). Novel mbtps2 missense mutation causes a keratosis follicularis spinulosa decalvans phenotype: mutation update and review of the literature. Clinical and Experimental Dermatology, 41(7), 757-760. https://doi.org/10.1111/ced.12889