Associated References
Main Article
Aetiology of Gout
Gout is primarily caused by hyperuricemia, a condition characterized by elevated levels of uric acid in the blood. The aetiology of gout involves both genetic and environmental factors. Genetic predisposition plays a significant role, with various studies identifying multiple risk loci associated with gout susceptibility, including single-nucleotide polymorphisms in genes related to uric acid metabolism, such as ABCG2 and SLC2A9 (Yamamoto et al., 2016; Phipps‐Green et al., 2016). Environmental factors, particularly dietary habits, significantly influence uric acid levels. Diets high in purines, such as those rich in red meat, seafood, and alcohol, contribute to increased uric acid production (Roddy & Choi, 2014; Xia et al., 2018). Additionally, conditions such as obesity, metabolic syndrome, and chronic kidney disease exacerbate the risk of developing hyperuricemia and, consequently, gout (Zhu et al., 2021; Pakpoor et al., 2015).
Prevalence of Gout
Recent epidemiological studies indicate a rising prevalence of gout globally, with significant variations across different populations. A systematic review highlighted that the prevalence of gout has increased over the past few decades, particularly in developed countries, where it affects approximately 1-4% of the adult population (Nicholson et al., 2018; Kuo et al., 2016). In specific cohorts, such as HIV-positive individuals, the prevalence can be even higher due to associated metabolic disturbances (Nicholson et al., 2018). Furthermore, a meta-analysis revealed that the prevalence of gout is notably higher in men compared to women, with a ratio of approximately 3:1, and it increases with age, particularly in those over 65 years (Roddy & Choi, 2014; Zhu et al. (2021).
Pathophysiological Changes in Gout
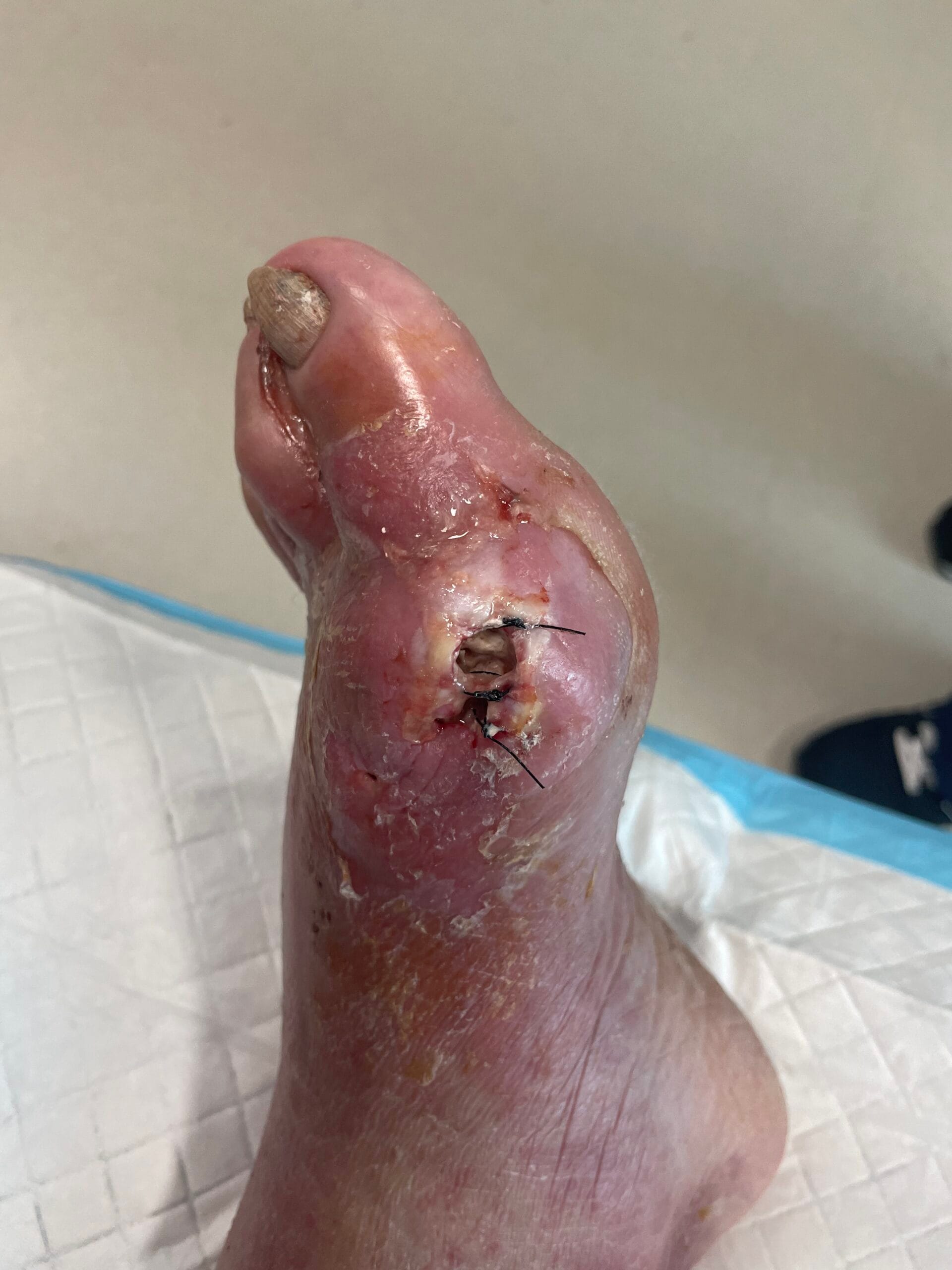
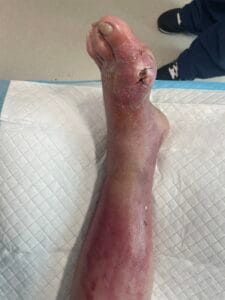
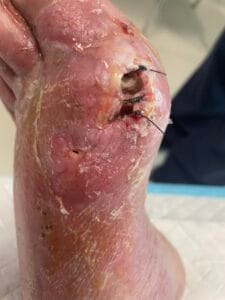
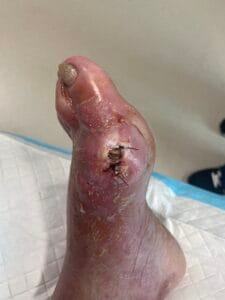
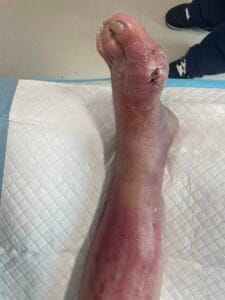
The pathophysiology of gout is primarily driven by the deposition of monosodium urate (MSU) crystals in joints and soft tissues, resulting from prolonged hyperuricemia. When uric acid levels exceed the solubility threshold, MSU crystals form and trigger an inflammatory response mediated by the NLRP3 inflammasome, leading to the release of pro-inflammatory cytokines such as interleukin-1 beta (IL-1β) (Holzinger et al., 2014; Ren et al., 2016). This inflammatory cascade results in acute gout flares characterized by intense pain, swelling, and redness in affected joints. Chronic gout can lead to the formation of tophi, which are aggregates of MSU crystals that can cause joint damage and deformity if left untreated (Bursill et al., 2019; Pérez-Ruiz et al., 2015). Additionally, the presence of comorbidities such as cardiovascular diseases further complicates the pathophysiological landscape of gout (Kuo et al., 2016; Zhu et al., 2021).
Evidence-Based Treatment Options
Current treatment strategies for gout focus on both acute management of flares and long-term urate-lowering therapy. Nonsteroidal anti-inflammatory drugs (NSAIDs), colchicine, and corticosteroids are commonly used for acute attacks (Paul & James, 2017; Robinson, 2018). For chronic management, urate-lowering therapies such as allopurinol and febuxostat are recommended to maintain serum uric acid levels below 6 mg/dL (Robinson, 2018; Zhu et al., 2021). Recent advancements have introduced novel agents like lesinurad and pegloticase, which provide additional options for patients who are refractory to traditional therapies (Paul & James, 2017; Robinson, 2018). Evidence suggests that early intervention and sustained urate-lowering therapy can significantly reduce the frequency of acute attacks and prevent long-term joint damage (Pérez-Ruiz et al., 2015; Zhu et al., 2021).
Precautions with Treatments
While effective, gout treatments come with specific precautions and contraindications. For instance, allopurinol can cause hypersensitivity reactions, including severe skin reactions, particularly in patients with renal impairment or those with a history of allopurinol hypersensitivity (Robinson, 2018; Zhu et al., 2021). Colchicine, while effective for acute flares, can lead to gastrointestinal side effects and should be used cautiously in patients with renal or hepatic dysfunction (Paul & James, 2017; Robinson, 2018). Additionally, the introduction of urate-lowering therapy during an acute attack may exacerbate symptoms, thus it is generally recommended to wait until the flare resolves before initiating such treatment (Robinson, 2018; Zhu et al., 2021).
Diagnostic Tests Available
The diagnosis of gout is primarily based on clinical presentation and the identification of MSU crystals in synovial fluid or tophaceous material. The gold standard for diagnosis is the detection of negatively birefringent MSU crystals under polarized light microscopy (Davies et al., 2019; Bursill et al., 2019). However, this procedure can be technically challenging and uncomfortable for patients. Other diagnostic methods include serum uric acid measurement, imaging techniques such as ultrasound and dual-energy computed tomography (DECT), which can help visualize urate deposits in joints and soft tissues (Davies et al., 2019; Jacobson et al., 2017). Elevated serum uric acid levels, while suggestive, are not definitive for gout diagnosis, as many individuals with hyperuricemia do not develop gout (Zhu et al., 2021; Phipps‐Green et al., 2016).
Contributing Factors
Gout is influenced by a variety of modifiable and non-modifiable risk factors. Modifiable factors include dietary choices, alcohol consumption, obesity, and certain medications such as diuretics, which can elevate uric acid levels (Roddy & Choi, 2014; Xia et al., 2018). Non-modifiable factors encompass genetic predisposition, age, and sex, with men being more susceptible than women, particularly before menopause (Nicholson et al., 2018; Zhu et al., 2021). Understanding these contributing factors is crucial for clinicians to implement effective prevention strategies and tailor treatment plans for individuals at risk of developing gout.
Conclusion
In summary, gout is a complex condition with multifactorial aetiology involving genetic, environmental, and lifestyle factors. The rising prevalence of gout necessitates a comprehensive understanding of its pathophysiology, diagnostic methods, and treatment options among health clinicians. By focusing on evidence-based practices and recognizing the importance of patient education regarding modifiable risk factors, healthcare professionals can significantly improve the management and outcomes for patients with gout.
References:
- Bursill, D., Taylor, W., Terkeltaub, R., Kuwabara, M., Merriman, T., Grainger, R., … & Dalbeth, N. (2019). Gout, hyperuricemia, and crystal‐associated disease network consensus statement regarding labels and definitions for disease elements in gout. Arthritis Care & Research, 71(3), 427-434. https://doi.org/10.1002/acr.23607
- Davies, J., Riede, P., Langevelde, K., & Teh, J. (2019). Recent developments in advanced imaging in gout. Therapeutic Advances in Musculoskeletal Disease, 11. https://doi.org/10.1177/1759720×19844429
- Holzinger, D., Nippe, N., Vogl, T., Marketon, K., Mysore, V., Weinhage, T., … & Roth, J. (2014). Myeloid‐related proteins 8 and 14 contribute to monosodium urate monohydrate crystal–induced inflammation in gout. Arthritis & Rheumatology, 66(5), 1327-1339. https://doi.org/10.1002/art.38369
- Jacobson, J., Ruangchaijatuporn, T., Khoury, V., & Magerkurth, O. (2017). Ultrasound of the knee: common pathology excluding extensor mechanism. Seminars in Musculoskeletal Radiology, 21(02), 102-112. https://doi.org/10.1055/s-0037-1599204
- Kuo, C., Grainge, M., Mallen, C., Zhang, W., & Doherty, M. (2016). Comorbidities in patients with gout prior to and following diagnosis: case-control study. Annals of the Rheumatic Diseases, 75(1), 210-217. https://doi.org/10.1136/annrheumdis-2014-206410
- Nicholson, P., Saunsbury, E., D’Angelo, S., Churchill, D., & Walker‐Bone, K. (2018). Prevalence of and risk factors for gout in hiv-positive adults: a case–control study. International Journal of STD & Aids, 30(3), 249-255. https://doi.org/10.1177/0956462418799803
- Pakpoor, J., Seminog, O., Ramagopalan, S., & Goldacre, M. (2015). Clinical associations between gout and multiple sclerosis, parkinson’s disease and motor neuron disease: record-linkage studies. BMC Neurology, 15(1). https://doi.org/10.1186/s12883-015-0273-9
- Paul, B. and James, R. (2017). Gout: an asia‐pacific update. International Journal of Rheumatic Diseases, 20(4), 407-416. https://doi.org/10.1111/1756-185x.13103
- Phipps‐Green, A., Merriman, M., Topless, R., Altaf, S., Montgomery, G., Franklin, C., … & Merriman, T. (2016). Twenty-eight loci that influence serum urate levels: analysis of association with gout. Annals of the Rheumatic Diseases, 75(1), 124-130. https://doi.org/10.1136/annrheumdis-2014-205877
- Pérez-Ruiz, F., Marimon, E., & Chinchilla, S. (2015). Hyperuricaemia with deposition: latest evidence and therapeutic approach. Therapeutic Advances in Musculoskeletal Disease, 7(6), 225-233. https://doi.org/10.1177/1759720×15599734
- Ren, Y., Jin, T., Sun, X., Geng, T., Zhang, M., Wang, L., … & Chen, C. (2016). Pdk2 and abcg2 genes polymorphisms are correlated with blood glucose levels and uric acid in tibetan gout patients. Genetics and Molecular Research, 15(1). https://doi.org/10.4238/gmr.15017447
- Robinson, P. (2018). Gout – an update of aetiology, genetics, co-morbidities and management. Maturitas, 118, 67-73. https://doi.org/10.1016/j.maturitas.2018.10.012
- Roddy, E. and Choi, H. (2014). Epidemiology of gout. Rheumatic Disease Clinics of North America, 40(2), 155-175. https://doi.org/10.1016/j.rdc.2014.01.001
- Xia, Y., Qi, X., Gu, Y., Jia, S., Qing, Z., Liu, L., … & Niu, K. (2018). A dietary pattern rich in animal organ, seafood and processed meat products is associated with newly diagnosed hyperuricaemia in chinese adults: a propensity score-matched case–control study. British Journal of Nutrition, 119(10), 1177-1184. https://doi.org/10.1017/s0007114518000867
- Yamamoto, K., Nakaoka, H., Nakayama, A., Sakiyama, M., Chiba, T., Takahashi, A., … & Shinomiya, N. (2016). Genome-wide association study of clinically defined gout identifies multiple risk loci and its association with clinical subtypes. Annals of the Rheumatic Diseases, 75(4), 652-659. https://doi.org/10.1136/annrheumdis-2014-206191
- Zhu, C., Sun, B., Zhang, B., & Zhou, Z. (2021). An update of genetics, co‐morbidities and management of hyperuricaemia. Clinical and Experimental Pharmacology and Physiology, 48(10), 1305-1316. https://doi.org/10.1111/1440-1681.13539