Associated References
Main Article
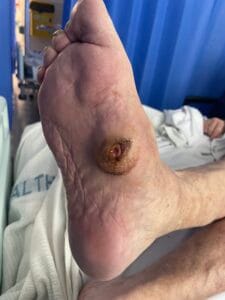
Aetiology of Diabetic Foot Ulcers
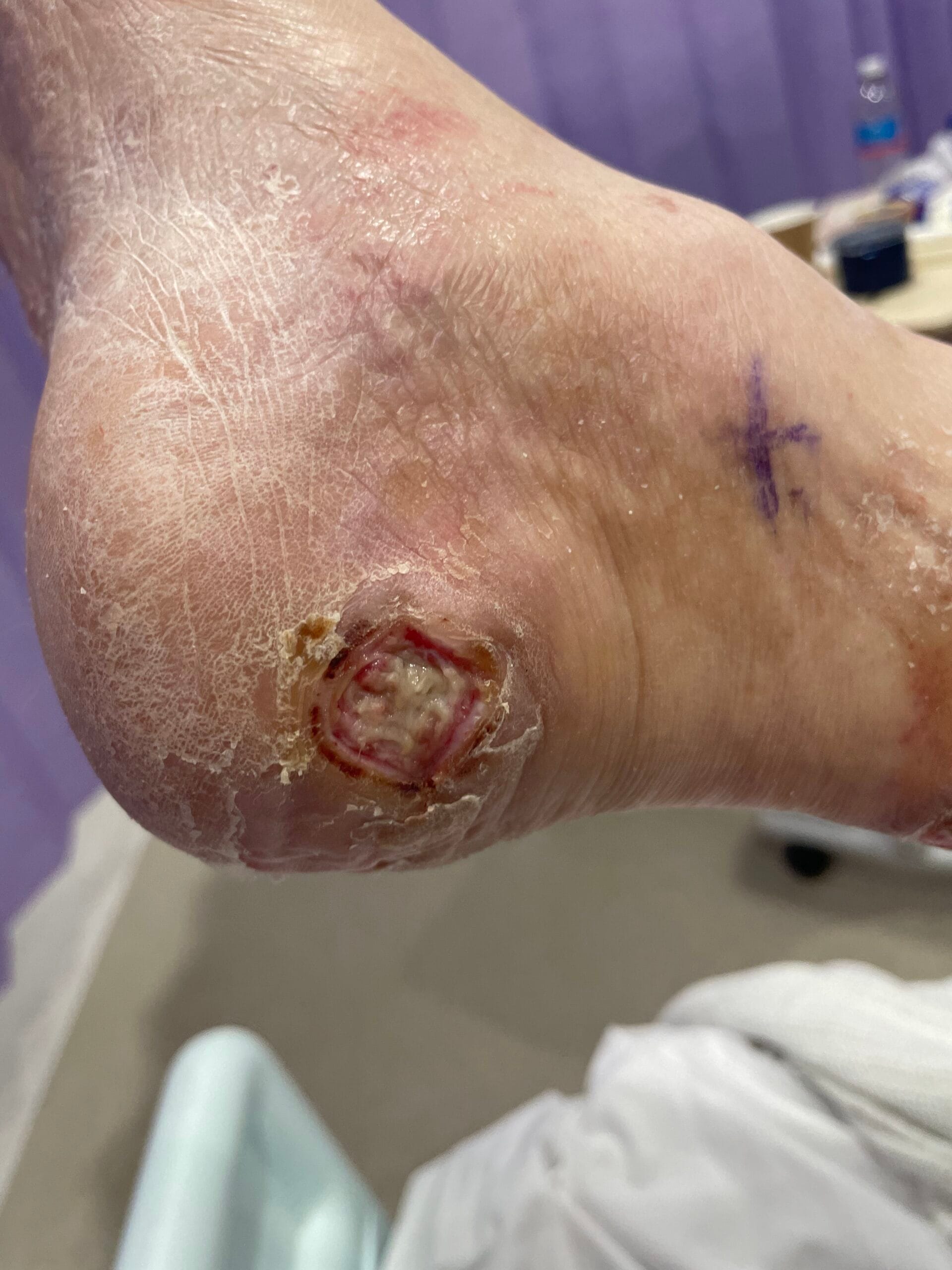
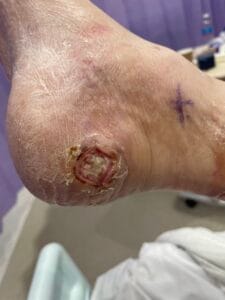
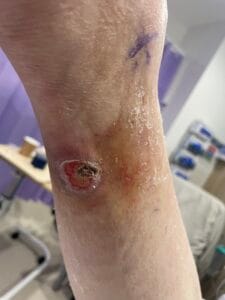
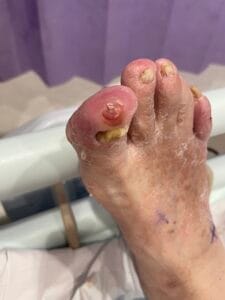
Diabetic foot ulcers (DFUs) are a significant complication of diabetes mellitus, primarily arising from a combination of neuropathy, ischemia, and infection. Neuropathy, particularly peripheral neuropathy, leads to a loss of protective sensation in the feet, making patients unaware of injuries or pressure points that can develop into ulcers (Saluja et al., 2019; Xia et al., 2018). Ischemia, often due to peripheral arterial disease (PAD), further exacerbates the risk by reducing blood flow to the extremities, impairing wound healing and increasing susceptibility to infections (Elgzyri et al., 2014; Bowling et al., 2015). Additionally, factors such as foot deformities, poor glycemic control, and inadequate foot care contribute to the development of DFUs (Markakis et al., 2016; Fu et al., 2019).
The multifactorial nature of DFUs necessitates a comprehensive understanding of these underlying causes, as they interact to create an environment conducive to ulceration. Chronic inflammation associated with diabetes also plays a role, as it can lead to tissue damage and impaired healing processes (Saluja et al., 2019; Fu et al., 2019). Furthermore, lifestyle factors such as smoking and obesity can exacerbate these conditions, highlighting the importance of addressing modifiable risk factors in patient management (Xia et al., 2018; Markakis et al., 2016).
Prevalence of Diabetic Foot Ulcers
Recent studies indicate that the prevalence of DFUs among diabetic patients ranges from 1.7% to 11.9%, with a lifetime incidence estimated at approximately 25% (Zhao et al., 2014; Dovell et al., 2021). The global burden of DFUs is substantial, with significant variations observed based on geographic and demographic factors. For instance, the prevalence in certain regions of Asia remains alarmingly high, reflecting both the rising incidence of diabetes and inadequate healthcare resources (Xia et al., 2018).
Moreover, the recurrence rate of DFUs is notably high, with studies suggesting that up to 40% of patients may experience a recurrence within a year following healing (Fu et al., 2019; Dovell et al., 2021). This underscores the critical need for ongoing monitoring and preventive strategies in individuals with a history of foot ulcers.
Pathophysiological Changes in Diabetic Foot Ulcers
The pathophysiology of DFUs involves complex interactions between neuropathy, ischemia, and infection. Neuropathy leads to a loss of sensation, which can result in unrecognized trauma and subsequent ulcer formation (Siersma et al., 2013; Xia et al., 2018). Ischemia, often due to PAD, compromises the blood supply necessary for healing and increases the risk of infection (Elgzyri et al., 2014; Bowling et al., 2015).
In addition, the impaired angiogenesis characteristic of diabetic patients further complicates the healing process. Studies have shown that mesenchymal stem cells (MSCs) can promote angiogenesis and enhance wound healing, suggesting potential therapeutic avenues (O’Loughlin et al., 2013; Zhao et al., 2014). The presence of chronic inflammation, characterized by elevated cytokines and immune dysregulation, also contributes to the delayed healing seen in DFUs (Saluja et al., 2019; Fu et al., 2019).
Evidence-Based Treatment Options
Current evidence-based treatments for DFUs include offloading, advanced wound dressings, and the use of growth factors and cellular therapies. Offloading is considered the cornerstone of treatment for neuropathic ulcers, as it reduces pressure on the affected area, facilitating healing (Markakis et al., 2016; Mulder et al., 2014). Advanced dressings, such as hydrocolloids and alginates, provide a moist wound environment conducive to healing and can help manage exudate (Mulder et al., 2014).
Additionally, the application of negative-pressure wound therapy has shown promise in promoting healing in complex DFUs (Markakis et al., 2016). Emerging therapies, including the use of MSCs and bioengineered skin substitutes, are also being explored, with some studies indicating improved healing rates compared to traditional methods (O’Loughlin et al., 2013; Zhao et al., 2014; Mulder et al., 2014).
Precautions with Treatments
While various treatment modalities exist, clinicians must be aware of specific precautions and contraindications. For instance, negative-pressure wound therapy may not be suitable for wounds with exposed organs, tendons, bone or in patients with certain types of malignancies (Markakis et al., 2016). Similarly, the use of growth factors and cellular therapies requires careful patient selection, as these treatments may not be effective in the presence of significant ischemia or infection (O’Loughlin et al., 2013; Mulder et al., 2014). A thorough neurovascular assessment always needs to take place with patients with a DFU as this will determine the type of dressing used. This is due to the patient’s inability to feel if their lower limb is becoming ischemic due to a circumferential dressing or compression that has been applied.
Furthermore, clinicians should monitor for potential adverse effects associated with advanced therapies, such as allergic reactions or complications from surgical interventions (Markakis et al., 2016; Mulder et al., 2014). A thorough assessment of the patient’s overall health status and comorbidities is essential to tailor treatment plans effectively.
Diagnostic Tests Available
The diagnosis of DFUs typically involves a comprehensive clinical assessment, including the evaluation of foot sensation, blood flow, and the presence of infection. Common diagnostic tests include the monofilament test for sensory neuropathy, Doppler ultrasound for assessing blood flow, and imaging studies to evaluate for osteomyelitis (Monteiro‐Soares et al., 2020; Crawford et al., 2018).
Additionally, laboratory tests may be conducted to assess for infection, including wound cultures and inflammatory markers (Lipsky et al., 2016; Uçkay et al., 2013). The use of classification systems, such as the SINBAD system, can aid in stratifying patients based on risk factors and guiding treatment decisions (Monteiro‐Soares et al., 2020).
Contributing Factors to Diabetic Foot Ulcers
Several modifiable and non-modifiable factors contribute to the risk of developing DFUs. Non-modifiable factors include age, duration of diabetes, and genetic predisposition (Xia et al., 2018; Dovell et al., 2021). In contrast, modifiable factors encompass lifestyle choices such as smoking, obesity, and poor glycemic control, which can significantly influence ulcer development and healing (Xia et al., 2018; Markakis et al., 2016).
Education and self-care practices are crucial in managing these risk factors. Intensive nursing education has been shown to reduce the incidence of DFUs among high-risk patients, emphasizing the importance of patient engagement in their care (Ren et al., 2014; Markakis et al., 2016). Regular foot examinations and appropriate footwear can also mitigate risks associated with foot injuries and ulceration (Bowling et al., 2015; Markakis et al., 2016).
Charcot Foot and the Requirement for a Multidisciplinary Team Approach
Charcot foot, also known as Charcot neuroarthropathy, is a serious complication of diabetes characterized by progressive degeneration of the bones and joints in the foot due to neuropathy. This condition often leads to significant deformities, instability, and an increased risk of ulceration and amputation if not managed appropriately (Rogers et al., 2011; Petrova & Edmonds, 2016). The pathophysiology of Charcot foot involves a combination of factors, including loss of protective sensation, increased mechanical stress on the foot, and inflammatory processes that contribute to bone resorption and joint destruction (Rogers et al., 2011; Petrova & Edmonds, 2016).
The management of Charcot foot is complex and necessitates a multidisciplinary team approach to optimize outcomes. This team typically includes endocrinologists, podiatrists, orthopedic surgeons, wound care specialists, and diabetes educators, all of whom play a vital role in the comprehensive care of affected patients (Kavros, 2012; Huang et al., 2012). The integration of various specialties is essential for addressing the multifaceted nature of the condition, which encompasses not only the orthopedic aspects but also the metabolic and systemic implications of diabetes (Acker et al., 2014; MacRury et al., 2018).
Early diagnosis and intervention are critical in the management of Charcot foot. Patients often present with swelling, warmth, and redness in the foot, which can be mistaken for infection or other conditions. Therefore, a high index of suspicion is required, particularly in patients with diabetes and neuropathy (Rogers et al., 2011; Diacogiorgis et al., 2021). Imaging studies, such as X-rays and MRI, are crucial for confirming the diagnosis and assessing the extent of bone and joint involvement (Chantelau & G, 2014; Pakarinen et al., 2011).
Once diagnosed, the initial management of Charcot foot typically involves offloading the affected foot to prevent further injury and allow for healing. This can be achieved through the use of total contact casts, specialized footwear, or orthotic devices (Kavros, 2012; Lowery et al., 2012). In some cases, surgical intervention may be necessary, particularly if there is recurrent ulceration, severe deformity, or infection (Kavros, 2012; Kucera et al., 2014). Surgical options may include arthrodesis, exostectomy, or even amputation in severe cases (Kavros, 2012; Kucera et al., 2014).
The role of the multidisciplinary team extends beyond immediate management; it also includes ongoing education and support for patients. Effective patient education regarding foot care, glycemic control, and the importance of regular foot examinations is essential in preventing complications associated with Charcot foot (Acker et al., 2014; MacRury et al., 2018). Furthermore, addressing psychosocial factors and providing emotional support can significantly enhance patient adherence to treatment and improve overall outcomes (MacRury et al., 2018; Kucera et al., 2014).
Research has shown that the implementation of multidisciplinary foot care teams can lead to a reduction in the incidence of foot ulcerations and amputations, as well as improved limb salvage rates (Acker et al., 2014; MacRury et al., 2018). A systematic review highlighted the disparities in the implementation of guidelines for diabetic foot care across Europe, emphasizing the need for standardized protocols and increased awareness among healthcare providers (Acker et al., 2014).
Charcot foot represents a significant challenge in the management of diabetic complications. A multidisciplinary team approach is essential for effective diagnosis, treatment, and prevention of complications. By fostering collaboration among various healthcare professionals, clinicians can provide comprehensive care that addresses the complex needs of patients with Charcot foot, ultimately improving their quality of life and reducing the burden of diabetes-related foot complications.
Conclusion
In summary, diabetic foot ulcers represent a complex interplay of various factors, necessitating a multifaceted approach to prevention and management. Understanding the aetiology, prevalence, pathophysiological changes, treatment options, and contributing factors is essential for healthcare professionals involved in the care of diabetic patients. By implementing evidence-based practices and addressing modifiable risk factors, clinicians can significantly improve outcomes for individuals at risk of developing DFUs.
References:
- Acker, K., Léger, P., Hartemann, A., Chawla, A., & Siddiqui, M. (2014). Burden of diabetic foot disorders, guidelines for management and disparities in implementation in europe: a systematic literature review. Diabetes/Metabolism Research and Reviews, 30(8), 635-645. https://doi.org/10.1002/dmrr.2523
- Bowling, F., Rashid, S., & Boulton, A. (2015). Preventing and treating foot complications associated with diabetes mellitus. Nature Reviews Endocrinology, 11(10), 606-616. https://doi.org/10.1038/nrendo.2015.130
- Chantelau, E. and G, G. (2014). Is the eichenholtz classification still valid for the diabetic charcot foot?. Swiss Medical Weekly. https://doi.org/10.4414/smw.2014.13948
- Crawford, F., Cézard, G., & Chappell, F. (2018). The development and validation of a multivariable prognostic model to predict foot ulceration in diabetes using a systematic review and individual patient data meta‐ Diabetic Medicine, 35(11), 1480-1493. https://doi.org/10.1111/dme.13797
- Diacogiorgis, D., Perrin, B., & Kingsley, M. (2021). Factors impacting the evidence‐based assessment, diagnosis and management of acute charcot neuroarthropathy: a systematic review. Journal of Foot and Ankle Research, 14(1). https://doi.org/10.1186/s13047-021-00469-5
- Dovell, G., Staniszewska, A., Ramirez, J., Murray, I., Ambler, G., Twine, C., … & Hinchliffe, R. (2021). A systematic review of outcome reporting for interventions to treat people with diabetic foot ulceration. Diabetic Medicine, 38(10). https://doi.org/10.1111/dme.14664
- Elgzyri, T., Larsson, J., Nyberg, P., Thörne, J., Eriksson, K., & Apelqvist, J. (2014). Early revascularization after admittance to a diabetic foot center affects the healing probability of ischemic foot ulcer in patients with diabetes. European Journal of Vascular and Endovascular Surgery, 48(4), 440-446. https://doi.org/10.1016/j.ejvs.2014.06.041
- Fu, X., Ding, H., Miao, W., Mao, C., Zhan, M., & Chen, H. (2019). Global recurrence rates in diabetic foot ulcers: a systematic review and meta‐ Diabetes/Metabolism Research and Reviews, 35(6). https://doi.org/10.1002/dmrr.3160
- Huang, Y., Lin, K., Yin, J., Chang, C., Chung, C., Chuang, L., … & Shin, S. (2012). Diabetes-related kidney, eye, and foot disease in taiwan: an analysis of the nationwide data for 2000–2009. Journal of the Formosan Medical Association, 111(11), 637-644. https://doi.org/10.1016/j.jfma.2012.09.006
- Kavros, S. (2012). Acellular fetal bovine dermal matrix for treatment of chronic ulcerations of the midfoot associated with charcot neuroarthropathy. Foot & Ankle Specialist, 5(4), 230-234. https://doi.org/10.1177/1938640012449037
- Kucera, T., Sponer, P., & Šrot, J. (2014). Surgical reconstruction of charcot foot neuroarthropathy, a case based review. Acta Medica (Hradec Kralove Czech Republic), 57(3), 127-132. https://doi.org/10.14712/18059694.2014.51
- Lipsky, B., Aragón‐Sánchez, J., Diggle, M., Embil, J., Kono, S., Lavery, L., … & Peters, E. (2016). Iwgdf guidance on the diagnosis and management of foot infections in persons with diabetes. Diabetes/Metabolism Research and Reviews, 32(S1), 45-74. https://doi.org/10.1002/dmrr.2699
- Lowery, N., Woods, J., Armstrong, D., & Wukich, D. (2012). Surgical management of charcot neuroarthropathy of the foot and ankle: a systematic review. Foot & Ankle International, 33(2), 113-121. https://doi.org/10.3113/fai.2012.0113
- MacRury, S., Stephen, K., Main, F., Gorman, J., Jones, S., & Macfarlane, D. (2018). Reducing amputations in people with diabetes (rapid): evaluation of a new care pathway. International Journal of Environmental Research and Public Health, 15(5), 999. https://doi.org/10.3390/ijerph15050999
- Markakis, K., Bowling, F., & Boulton, A. (2016). The diabetic foot in 2015: an overview. Diabetes/Metabolism Research and Reviews, 32(S1), 169-178. https://doi.org/10.1002/dmrr.2740
- Monteiro‐Soares, M., Boyko, E., Jeffcoate, W., Mills, J., Russell, D., Morbach, S., … & Game, F. (2020). Diabetic foot ulcer classifications: a critical review. Diabetes/Metabolism Research and Reviews, 36(S1). https://doi.org/10.1002/dmrr.3272
- Monteiro‐Soares, M., Russell, D., Boyko, E., Jeffcoate, W., Mills, J., Morbach, S., … & Game, F. (2020). Guidelines on the classification of diabetic foot ulcers (iwgdf 2019). Diabetes/Metabolism Research and Reviews, 36(S1). https://doi.org/10.1002/dmrr.3273
- Mulder, G., Tenenhaus, M., & D’Souza, G. (2014). Reduction of diabetic foot ulcer healing times through use of advanced treatment modalities. The International Journal of Lower Extremity Wounds, 13(4), 335-346. https://doi.org/10.1177/1534734614557925
- O’Loughlin, A., Kulkarni, M., Creane, M., Vaughan, E., Mooney, E., Shaw, G., … & O’Brien, T. (2013). Topical administration of allogeneic mesenchymal stromal cells seeded in a collagen scaffold augments wound healing and increases angiogenesis in the diabetic rabbit ulcer. Diabetes, 62(7), 2588-2594. https://doi.org/10.2337/db12-1822
- Pakarinen, T., Laine, H., Mäenpää, H., Mattila, P., & Lahtela, J. (2011). The effect of zoledronic acid on the clinical resolution of charcot neuroarthropathy. Diabetes Care, 34(7), 1514-1516. https://doi.org/10.2337/dc11-0396
- Petrova, N. and Edmonds, M. (2016). Acute charcot neuro‐ Diabetes/Metabolism Research and Reviews, 32(S1), 281-286. https://doi.org/10.1002/dmrr.2734
- Ren, M., Yang, C., Lin, D., Xiao, H., Li, F., Guo, Y., … & Li, Y. (2014). Effect of intensive nursing education on the prevention of diabetic foot ulceration among patients with high-risk diabetic foot: a follow-up analysis. Diabetes Technology & Therapeutics, 16(9), 576-581. https://doi.org/10.1089/dia.2014.0004
- Rogers, L., Frykberg, R., Armstrong, D., Boulton, A., Edmonds, M., Van, G., … & Uccioli, L. (2011). The charcot foot in diabetes. Diabetes Care, 34(9), 2123-2129. https://doi.org/10.2337/dc11-0844
- Saluja, S., Anderson, S., Hambleton, I., Shoo, H., Livingston, M., Jude, E., … & Heald, A. (2019). Foot ulceration and its association with mortality in diabetes mellitus: a meta‐ Diabetic Medicine, 37(2), 211-218. https://doi.org/10.1111/dme.14151
- Siersma, V., Thorsen, H., Holstein, P., Kars, M., Apelqvist, J., Jude, E., … & Schaper, N. (2013). Importance of factors determining the low health‐related quality of life in people presenting with a diabetic foot ulcer: the eurodiale study. Diabetic Medicine, 30(11), 1382-1387. https://doi.org/10.1111/dme.12254
- Uçkay, İ., Gariani, K., Pataky, Z., & Lipsky, B. (2013). Diabetic foot infections: state‐of‐the‐ Diabetes Obesity and Metabolism, 16(4), 305-316. https://doi.org/10.1111/dom.12190
- Xia, N., Morteza, A., Yang, F., Cao, H., & Wang, A. (2018). Review of the role of cigarette smoking in diabetic foot. Journal of Diabetes Investigation, 10(2), 202-215. https://doi.org/10.1111/jdi.12952
- Zhao, Q., Xia, N., Zhao, N., Li, M., Bi, C., Zhu, Q., … & Zhang, C. (2014). Localization of human mesenchymal stem cells from umbilical cord blood and their role in repair of diabetic foot ulcers in rats. International Journal of Biological Sciences, 10(1), 80-89. https://doi.org/10.7150/ijbs.7237