Associated References
Main Article
Aetiology of Crush Injury
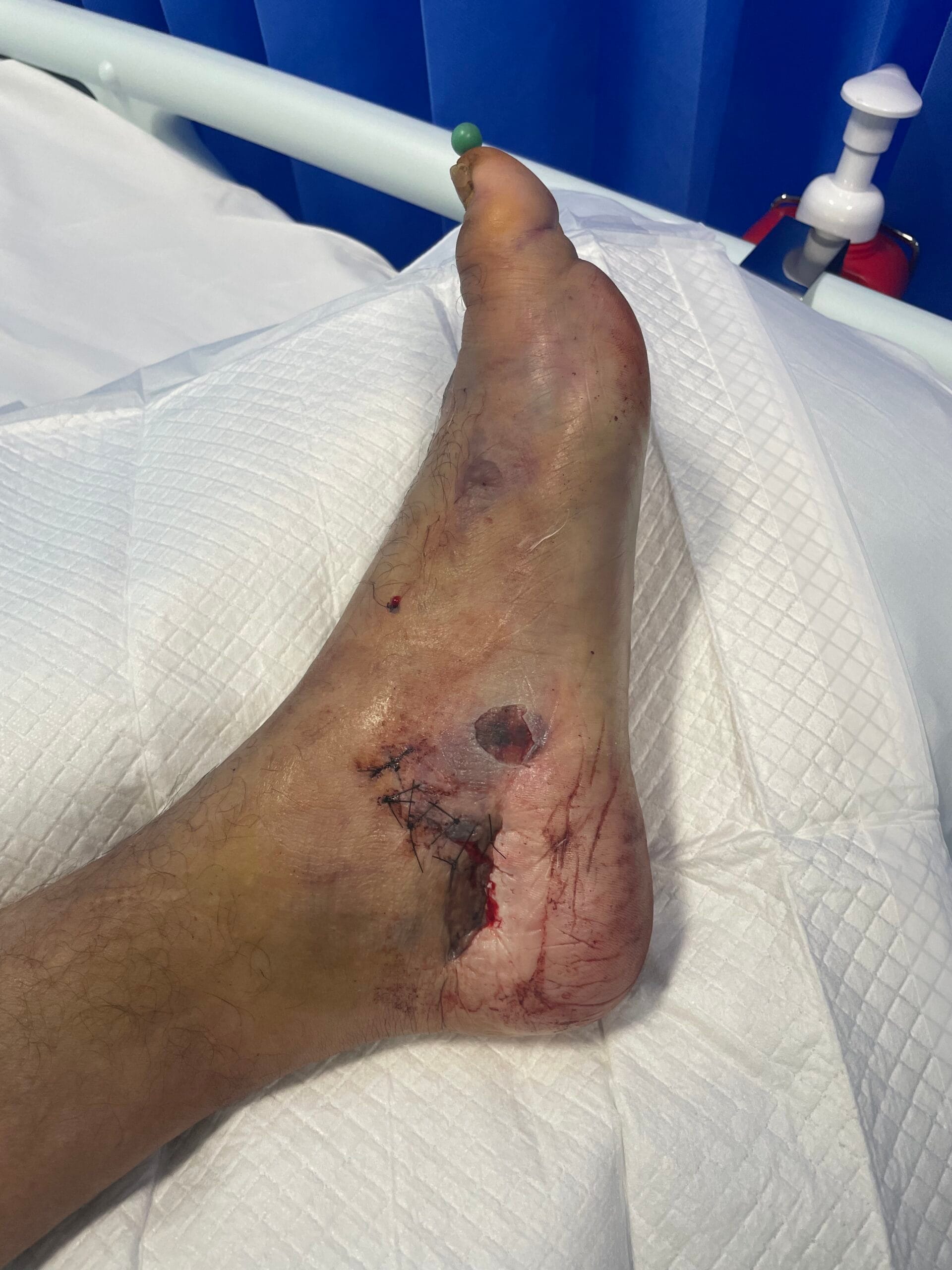
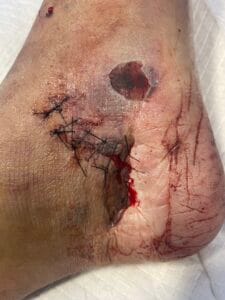
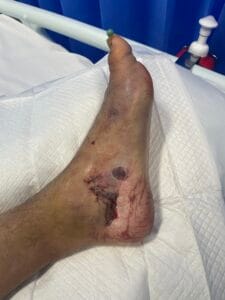
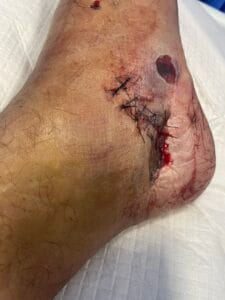
Crush injuries are primarily caused by the application of a significant force that compresses body tissues, leading to damage. Common scenarios include industrial accidents, vehicular collisions, and natural disasters, where individuals may become trapped under heavy objects or debris (Panesar & Ashkan, 2018). The mechanism of injury often involves blunt trauma, which can result in both soft tissue and bony injuries. Additionally, crush injuries can occur in specific contexts such as military operations or space exploration, where the risk of such injuries is heightened due to environmental factors and equipment failures (Panesar & Ashkan, 2018; Hussain et al., 2020).
The pathophysiological response to crush injuries is complex and involves multiple biological processes. For instance, the initial trauma can lead to immediate vascular compromise, resulting in ischemia and subsequent necrosis of the affected tissues (Chavez et al., 2016). Furthermore, the inflammatory response is triggered, characterized by the infiltration of macrophages and the release of pro-inflammatory cytokines, which can exacerbate tissue damage if not properly managed (Sawano et al., 2014; Poree et al., 2013).
Prevalence of Crush Injury
Recent studies indicate that crush injuries are a significant public health concern, particularly in occupational settings. For example, the prevalence of crush injuries in industrial environments can be as high as 20% of all reported workplace injuries (Chavez et al., 2016). Moreover, in the context of natural disasters, such as earthquakes, the incidence of crush injuries can dramatically increase, leading to a surge in emergency medical responses (Chavez et al., 2016; Furák & Athanassiadi, 2019).
In military settings, crush injuries are also prevalent, with reports indicating that they account for a substantial proportion of battlefield injuries (Gurjar et al., 2021). The overall incidence of crush injuries varies by region and activity, but it remains a critical area of focus for health clinicians, particularly in emergency and trauma care (Hussain et al., 2020; Chavez et al., 2016).
Pathophysiological Changes
The pathophysiological changes following a crush injury involve a cascade of cellular and molecular events. Initially, the mechanical trauma leads to direct cellular damage and disruption of vascular integrity, resulting in hemorrhage and edema (Chavez et al., 2016). This is followed by a complex inflammatory response, where macrophages play a pivotal role in tissue repair and regeneration (Sawano et al., 2014; Poree et al., 2013).
The release of growth factors such as hepatocyte growth factor (HGF) and nerve growth factor (NGF) is crucial for the regeneration of damaged tissues (Haggerty et al., 2019; Li et al., 2018). However, excessive inflammation can lead to secondary complications, including rhabdomyolysis and acute kidney injury, which are common sequelae of severe crush injuries (Chavez et al., 2016; Carvalho et al., 2020). The interplay between inflammation and tissue repair is critical, as it can determine the long-term outcomes for affected individuals (Haggerty et al., 2019; Li et al., 2018).
Treatments for Crush Injury
Current evidence-based treatment options for crush injuries focus on both immediate management and long-term rehabilitation. Immediate treatment often involves stabilization of the patient, addressing any life-threatening conditions, and managing pain (Chavez et al., 2016). Surgical intervention may be necessary to relieve pressure, repair damaged tissues, or amputate severely injured limbs (Hussain et al., 2020; Haggerty et al., 2019).
Rehabilitation plays a crucial role in recovery, with physical therapy being essential for restoring function and mobility (Carvalho et al., 2020). Additionally, pharmacological treatments, including the use of analgesics and anti-inflammatory medications, are commonly employed to manage pain and reduce inflammation (Bhusal et al., 2016; Nouri et al., 2018). Emerging therapies, such as low-level laser therapy and the use of biomaterials for nerve regeneration, are also being explored (Días et al., 2013; Li et al., 2018).
Precautions with Treatments
While treating crush injuries, clinicians must be aware of specific precautions and contraindications associated with various treatment modalities. For instance, the use of certain analgesics may be contraindicated in patients with renal impairment, which can be a concern in cases of rhabdomyolysis (Chavez et al., 2016; Carvalho et al., 2020).
Surgical interventions carry risks of infection and further tissue damage, necessitating careful patient selection and preoperative assessment (Haggerty et al., 2019; Carvalho et al., 2020). Moreover, rehabilitation strategies must be tailored to the individual, considering factors such as age, comorbidities, and the extent of the injury (Carvalho et al., 2020; Alaqeel & Alshomer, 2014).
Diagnostic Tests Available
The diagnosis of crush injuries typically involves a combination of clinical assessment and imaging studies. High-resolution ultrasound has emerged as a valuable tool for evaluating peripheral nerve injuries, providing detailed information about the extent of damage (Alaqeel & Alshomer, 2014). Additionally, magnetic resonance imaging (MRI) can be utilized to assess soft tissue injuries and identify any associated complications (Alaqeel & Alshomer, 2014; Furák & Athanassiadi, 2019).
Laboratory tests may also be necessary to evaluate renal function, particularly in cases where rhabdomyolysis is suspected (Chavez et al., 2016; Carvalho et al., 2020). Clinicians should employ a multimodal approach to ensure accurate diagnosis and effective management of crush injuries.
Contributing Factors
Several factors contribute to the risk of crush injuries, which can be categorized into modifiable and non-modifiable factors. Non-modifiable factors include age and underlying health conditions, such as diabetes, which can impair healing and increase the risk of complications (Gurjar et al., 2021; Carvalho et al., 2020).
Modifiable factors encompass workplace safety practices, equipment maintenance, and adherence to safety protocols, which can significantly reduce the incidence of crush injuries in occupational settings (Chavez et al., 2016; Furák & Athanassiadi, 2019). Education and training for individuals in high-risk environments are essential for minimizing the risk of such injuries (Hussain et al., 2020; Chavez et al., 2016).
Conclusion
Crush injuries represent a multifaceted challenge for health clinicians, requiring a comprehensive understanding of their aetiology, prevalence, pathophysiological changes, treatment options, and associated risks. By employing evidence-based practices and remaining vigilant about contributing factors, clinicians can enhance patient outcomes and mitigate the impact of these injuries on individuals and society.
References:
- Alaqeel, A. and Alshomer, F. (2014). High resolution ultrasound in the evaluation and management of traumatic peripheral nerve injuries: review of the literature. Oman Medical Journal, 29(5), 314-319. https://doi.org/10.5001/omj.2014.86
- Bhusal, S., Diomampo, S., & Magrey, M. (2016). Clinical utility, safety, and efficacy of pregabalin in the treatment of fibromyalgia. Drug Healthcare and Patient Safety, 13. https://doi.org/10.2147/dhps.s95535
- Carvalho, C., Reis, R., & Oliveira, J. (2020). Fundamentals and current strategies for peripheral nerve repair and regeneration., 173-201. https://doi.org/10.1007/978-981-15-3258-0_12
- Chavez, L., León, M., Einav, S., & Varon, J. (2016). Beyond muscle destruction: a systematic review of rhabdomyolysis for clinical practice. Critical Care, 20(1). https://doi.org/10.1186/s13054-016-1314-5
- Días, F., Issa, J., Iyomasa, M., Coutinho‐Netto, J., Calzzani, R., Iyomasa, D., … & Watanabe, I. (2013). Application of a low-level laser therapy and the purified protein from natural latex (hevea brasiliensis) in the controlled crush injury of the sciatic nerve of rats: a morphological, quantitative, and ultrastructural study. Biomed Research International, 2013, 1-11. https://doi.org/10.1155/2013/597863
- Furák, J. and Athanassiadi, K. (2019). Diaphragm and transdiaphragmatic injuries. Journal of Thoracic Disease, 11(S2), S152-S157. https://doi.org/10.21037/jtd.2018.10.76
- Gurjar, A., Manto, K., Estrada, J., Kaufman, M., Sun, D., Talukder, M., … & Elfar, J. (2021). 4-aminopyridine: a single-dose diagnostic agent to differentiate axonal continuity in nerve injuries. Military Medicine, 186(Supplement_1), 479-485. https://doi.org/10.1093/milmed/usaa310
- Haggerty, A., Bening, M., Pherribo, G., Dauer, E., & Oudega, M. (2019). Laminin polymer treatment accelerates repair of the crushed peripheral nerve in adult rats. Acta Biomaterialia, 86, 185-193. https://doi.org/10.1016/j.actbio.2019.01.024
- Hussain, G., Wang, J., Rasul, A., Anwar, H., Qasim, M., Zafar, S., … & Sun, T. (2020). Current status of therapeutic approaches against peripheral nerve injuries: a detailed story from injury to recovery. International Journal of Biological Sciences, 16(1), 116-134. https://doi.org/10.7150/ijbs.35653
- Li, R., Li, Y., Wu, Y., Zhao, Y., Chen, H., Yuan, Y., … & Xiao, J. (2018). Heparin-poloxamer thermosensitive hydrogel loaded with bfgf and ngf enhances peripheral nerve regeneration in diabetic rats. Biomaterials, 168, 24-37. https://doi.org/10.1016/j.biomaterials.2018.03.044
- Nouri, A., Patel, K., Montejo, J., Nasser, R., Gimbel, D., Sciubba, D., … & Cheng, J. (2018). The role of vitamin b12 in the management and optimization of treatment in patients with degenerative cervical myelopathy. Global Spine Journal, 9(3), 331-337. https://doi.org/10.1177/2192568218758633
- Panesar, S. and Ashkan, K. (2018). Surgery in space. British Journal of Surgery, 105(10), 1234-1243. https://doi.org/10.1002/bjs.10908
- Poree, L., Krames, E., Pope, J., Deer, T., Levy, R., & Schultz, L. (2013). Spinal cord stimulation as treatment for complex regional pain syndrome should be considered earlier than last resort therapy. Neuromodulation Technology at the Neural Interface, 16(2), 125-141. https://doi.org/10.1111/ner.12035
- Sawano, S., Suzuki, T., Q., M., Ohtsubo, H., Mizunoya, W., Ikeuchi, Y., … & Tatsumi, R. (2014). Supplementary immunocytochemistry of hepatocyte growth factor production in activated macrophages early in muscle regeneration. Animal Science Journal, 85(12), 994-1000. https://doi.org/10.1111/asj.12264