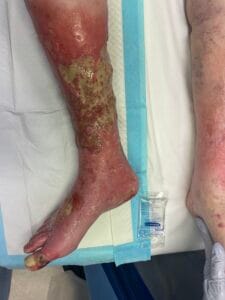
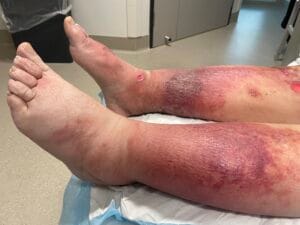
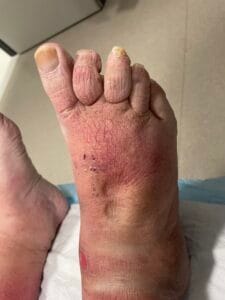
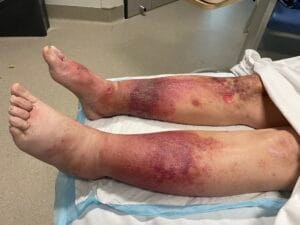
Quick Overview
Associated References
Main Article
History
The use of compression dressings in wound care has a rich history that dates back to ancient civilizations, where rudimentary forms of bandaging were employed to control bleeding and promote healing. Over the centuries, the understanding of wound management evolved significantly, particularly with the advent of modern medicine in the 19th century. The introduction of elastic bandages in the early 20th century marked a pivotal moment in the development of compression therapy. These bandages provided a controlled amount of pressure to the affected area, which was found to be beneficial in managing venous disorders and promoting wound healing (Sajjad et al., 2020).
In the latter half of the 20th century, advancements in materials science led to the development of more sophisticated compression systems, including multi-layer bandages and specialized compression garments. These innovations were driven by a growing body of evidence supporting the efficacy of compression in treating chronic venous insufficiency and venous leg ulcers (Brouwers et al., 2022). The evolution of compression dressings has continued into the 21st century, with the introduction of new technologies such as hydrocolloid and foam dressings that incorporate compression properties while also managing exudate and promoting a moist wound environment (Kayamori et al., 2016).
Mechanism of Action
Compression dressings work primarily by exerting controlled pressure on the wound and surrounding tissues, which plays a crucial role in enhancing venous return and reducing edema. The application of compression increases the hydrostatic pressure in the interstitial space, thereby facilitating the reabsorption of excess fluid into the venous system (Afghani & Cheraghali, 2021). This mechanism is particularly important in conditions such as chronic venous insufficiency, where impaired venous return leads to fluid accumulation and subsequent tissue swelling.
Moreover, compression dressings enhance wound healing by promoting local blood flow and oxygenation to the affected area. Increased pressure on the tissues stimulates the release of nitric oxide, a vasodilator that improves microcirculation and nutrient delivery to the wound site (Schuh, 2024). Additionally, the mechanical support provided by compression dressings can help stabilize the wound environment, reducing the risk of trauma and infection, which are critical factors in the healing process (Wu et al., 2017).
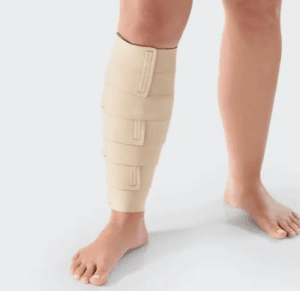
Compression dressings are classified into two primary categories based on their elasticity: short-stretch and long-stretch bandages. Each type has distinct mechanisms of action that contribute to their effectiveness in wound care and management of venous disorders.
Short-Stretch Compression
Short-stretch bandages are characterized by their low elasticity and high resistance to stretch. When applied, they exert a high level of pressure during muscle contraction, which is particularly beneficial for patients with venous insufficiency. This mechanism is crucial because it enhances venous return by promoting the muscular pump effect. As the muscles contract, the short-stretch bandage provides resistance, which helps to propel blood back toward the heart, thereby reducing venous hypertension and preventing the pooling of blood in the lower extremities Dabiri et al. (2013).
The effectiveness of short-stretch compression is further enhanced by its ability to maintain consistent pressure during activity. This is particularly important for patients who are mobile, as the bandage works in conjunction with muscle contractions to facilitate venous return. Studies have demonstrated that short-stretch bandages can significantly reduce edema and improve healing rates in patients with venous leg ulcers (Sajjad et al., 2020). Furthermore, the application of short-stretch bandages has been associated with lower recurrence rates of ulcers, highlighting their role in long-term management strategies for chronic venous insufficiency (Luthra et al., 2019).
Long-Stretch Compression
In contrast, long-stretch bandages possess a higher degree of elasticity, allowing them to stretch significantly when applied. This type of compression dressing exerts a lower resting pressure but can provide a higher working pressure when the patient is active. The mechanism of action for long-stretch bandages is primarily based on their ability to provide graduated compression. This means that the pressure exerted by the bandage decreases as it moves away from the heart, which is essential for promoting venous return and reducing edema (Pham et al., 2012).
Long-stretch bandages are particularly useful in situations where a higher level of compression is required, such as in the management of lymphedema or severe venous insufficiency. They can be applied in multiple layers to achieve the desired level of compression, which can be adjusted based on the patient’s specific needs. The sustained pressure provided by long-stretch bandages has been shown to enhance lymphatic drainage and improve overall circulation, thereby facilitating wound healing (Pham et al., 2012). Moreover, the ability to customize the level of compression allows healthcare professionals to tailor treatment to individual patient profiles, optimizing outcomes (Luthra et al., 2019).
Comparative Effectiveness
The choice between short-stretch and long-stretch compression bandages often depends on the specific clinical scenario and the patient’s condition. For instance, short-stretch bandages are generally preferred for patients with significant edema and those who are active, as they provide effective support during muscle contractions. Conversely, long-stretch bandages may be more suitable for patients requiring sustained pressure over extended periods, particularly in cases of severe venous insufficiency or lymphedema (Pham et al., 2012).
Research has indicated that both types of compression dressings can be effective in managing venous ulcers and other related conditions, but their mechanisms of action differ significantly. The selection of the appropriate type of dressing should be guided by clinical assessment, patient mobility, and the specific therapeutic goals (Pham et al., 2012). Ultimately, understanding the distinct mechanisms of action associated with short-stretch and long-stretch compression dressings enables healthcare professionals to make informed decisions that enhance patient care and improve healing outcomes.
Conclusion
In conclusion, the mechanisms of action of compression dressings, particularly short-stretch and long-stretch bandages, play a critical role in their effectiveness for wound care and management of venous disorders. Short-stretch bandages facilitate venous return through the muscular pump effect, while long-stretch bandages provide graduated compression that supports circulation and reduces edema. The choice of dressing should be tailored to the individual patient’s needs, taking into consideration their specific condition and mobility. By leveraging the unique properties of each type of compression dressing, healthcare professionals can optimize treatment strategies and improve patient outcomes.
Clinical Uses
Compression dressings are indicated for a variety of clinical conditions, most notably venous leg ulcers, lymphedema, and chronic venous insufficiency. Venous leg ulcers, which are often a consequence of chronic venous disease, benefit significantly from compression therapy as it helps to reduce edema and improve venous return, thereby facilitating healing (Chuter et al., 2023). Studies have shown that patients with venous leg ulcers treated with compression dressings experience higher healing rates compared to those receiving standard care (Wang et al., 2022).
In the case of lymphedema, compression dressings are utilized to manage swelling by promoting lymphatic drainage. The application of graduated compression helps to mobilize lymph fluid from the interstitial space back into the lymphatic system, thereby reducing limb volume and discomfort (Brekelmans et al., 2019). Furthermore, in patients with chronic venous insufficiency, compression therapy can alleviate symptoms such as pain and heaviness in the legs, enhancing the overall quality of life (Sonter et al., 2017).
Diagnostic Testing Requirements
Before initiating compression therapy, it is essential to conduct thorough diagnostic evaluations to ensure patient safety and treatment efficacy. The Ankle-Brachial Index (ABI) is a critical test used to assess peripheral arterial disease (PAD). It compares the blood pressure in the patient’s ankle with the blood pressure in the arm, providing valuable information about arterial circulation. An ABI of less than 0.9 is indicative of PAD, which contraindicates the use of high-compression dressings due to the risk of ischemia (Pinsornsak & Chumchuen, 2013).
In addition to ABI, Toe Pressures and Toe-Brachial Index (TBI) measurements are particularly important in diabetic patients. These tests assess microvascular health and can help identify patients at risk for poor wound healing. A TBI of less than 0.7 is often associated with a higher risk of complications and non-healing wounds, making it crucial to evaluate these parameters before applying compression therapy (Arora, 2023). The significance of these tests cannot be overstated, as they guide clinicians in making informed decisions regarding the appropriateness of compression treatment (Luthra et al., 2019).
Brands
Several brands of compression systems are widely used in clinical practice, each offering unique features tailored to specific patient needs. Profore, for instance, is a four-layer compression bandage system that provides sustained pressure and is particularly effective for managing venous leg ulcers (Zobel et al., 2017). Coban 2 is another popular option, known for its ease of application and ability to conform to various body contours while providing effective compression (Peltokangas et al., 2019). Jobst compression garments are also frequently utilized, especially for patients requiring long-term management of chronic venous conditions (Valle, 2024). Each of these systems has been validated through clinical studies demonstrating their effectiveness in promoting wound healing and managing edema (Baines, 2024).
Precautions
While compression dressings are beneficial, their application must be approached with caution. Contraindications include critical limb ischemia, where the risk of exacerbating tissue ischemia outweighs the potential benefits of compression (Mizzi et al., 2022). Additionally, complications such as skin necrosis, nerve compression, and discomfort can arise if the dressing is applied too tightly or improperly (Sadler et al., 2015). Regular monitoring of the patient’s condition is essential to ensure that the compression therapy remains effective and does not lead to adverse outcomes (Prasad et al., 2019). Clinicians should also educate patients about the signs of complications, such as increased pain, changes in skin color, or swelling beyond the area of compression, which may indicate the need for immediate reassessment (Koivunen et al., 2021).
Conclusion
In summary, compression dressings play a vital role in wound care and the management of various vascular conditions. Their historical evolution reflects advancements in medical understanding and technology, while their mechanisms of action underscore their importance in promoting healing and reducing complications. Clinicians must remain vigilant in assessing patients for appropriate diagnostic indicators before initiating therapy and be aware of the various brands available to tailor treatment to individual needs. By adhering to best practices and monitoring patient outcomes, healthcare professionals can optimize the use of compression dressings in their clinical practice.
References:
- Afghani, R. and Cheraghali, R. (2021). Below knee angioplasty results in diabetic aand non-diabetic patients. PJMHS, 15(6), 2063-2066. https://doi.org/10.53350/pjmhs211562063
- Arora, E. (2023). Correlation between toe brachial index and walking ability in peripheral arterial disease with type 2 diabetes mellitus. Bulletin of Faculty of Physical Therapy, 28(1). https://doi.org/10.1186/s43161-023-00155-6
- Baines, B. (2024). Temporal changes in toe-brachial index results in haemodialysis patients. Plos One, 19(4), e0301376. https://doi.org/10.1371/journal.pone.0301376
- Brekelmans, W., Burg, B., Vroom, M., Kreuger, M., Meer, A., & Hoencamp, R. (2019). Prevalence of foot ulcers in dialysis‐dependent patients. Wound Repair and Regeneration, 27(6), 687-692. https://doi.org/10.1111/wrr.12750
- Brouwers, J., Doorn, L., Pronk, L., Wissen, R., Putter, H., Schepers, A., … & Hamming, J. (2022). Doppler ultrasonography derived maximal systolic acceleration: value determination with artificially induced stenosis. Vascular and Endovascular Surgery, 56(5), 472-479. https://doi.org/10.1177/15385744221076269
- Chuter, V., Schaper, N., Hinchliffe, R., Mills, J., Azuma, N., Behrendt, C., … & Fitridge, R. (2023). Performance of non‐invasive bedside vascular testing in the prediction of wound healing or amputation among people with foot ulcers in diabetes: a systematic review. Diabetes/Metabolism Research and Reviews, 40(3). https://doi.org/10.1002/dmrr.3701
- Dabiri, G., Hammerman, S., Carson, P., & Falanga, V. (2013). Low‐grade elastic compression regimen for venous leg ulcers – an effective compromise for patients requiring daily dressing changes. International Wound Journal, 12(6), 655-661. https://doi.org/10.1111/iwj.12186
- Kayamori, S., Tsukada, S., Satô, M., Komata, K., Isida, Y., & Wakui, M. (2016). Impact of postoperative compression dressing using polyethylene foam pad on the multimodal protocol for swelling control following total knee arthroplasty: a randomized controlled trial. Arthroplasty Today, 2(4), 199-204. https://doi.org/10.1016/j.artd.2016.05.004
- Koivunen, V., Juonala, M., Venermo, M., Laivuori, M., Jalkanen, J., & Hakovirta, H. (2021). Toe pressure and toe brachial index are predictive of cardiovascular mortality regardless of the most diseased arterial segment in symptomatic lower-extremity artery disease—a retrospective cohort study. Plos One, 16(11), e0259122. https://doi.org/10.1371/journal.pone.0259122
- Luthra, L., Prasad, R., Kumar, R., Mitta, N., & Varghese, T. (2019). Role of four layer compression dressings in management of chronic venous ulcers. International Journal of Recent Surgical and Medical Sciences, 5(02), 42-45. https://doi.org/10.1055/s-0040-1708222
- Luthra, L., Prasad, R., Kumar, R., Mitta, N., & Varghese, T. (2019). Role of four layer compression dressings in management of chronic venous ulcers. International Journal of Recent Surgical and Medical Sciences, 5(02), 42-45. https://doi.org/10.1055/s-0040-1708222
- Mizzi, A., Cassar, K., Bowen, C., Camilleri, L., & Formosa, C. (2022). The impact of diabetes in intermittent claudication: a prospective cohort study. The International Journal of Lower Extremity Wounds. https://doi.org/10.1177/15347346221142189
- Peltokangas, M., Suominen, V., Vakhitov, D., Korhonen, J., Verho, J., Mattila, V., … & Oksala, N. (2019). Effects of percutaneous transluminal angioplasty of superficial femoral artery on photoplethysmographic pulse transit times. Ieee Journal of Biomedical and Health Informatics, 23(3), 1058-1065. https://doi.org/10.1109/jbhi.2018.2851388
- Pham, B., Harrison, M., Chen, M., & Carley, M. (2012). Cost-effectiveness of compression technologies for evidence-informed leg ulcer care: results from the canadian bandaging trial. BMC Health Services Research, 12(1). https://doi.org/10.1186/1472-6963-12-346
- Pinsornsak, P. and Chumchuen, S. (2013). Can a modified robert jones bandage after knee arthroplasty reduce blood loss? a prospective randomized controlled trial. Clinical Orthopaedics and Related Research, 471(5), 1677-1681. https://doi.org/10.1007/s11999-013-2786-0
- Prasad, R., Kamath, T., Ginsberg, C., Potok, O., Ix, J., Garimella, P., … & Rifkin, D. (2019). The association of the ankle‐brachial index, the toe‐brachial index, and their difference, with mortality and limb outcomes in dialysis patients. Hemodialysis International, 23(2), 214-222. https://doi.org/10.1111/hdi.12734
- Sadler, S., Hawke, F., & Chuter, V. (2015). The effect of pretest rest time on automated measures of toe systolic blood pressure and the toe brachial index. Blood Pressure Monitoring, 20(5), 245-248. https://doi.org/10.1097/mbp.0000000000000126
- Sajjad, A., Kala, S., Gupta, A., & Varshney, A. (2020). Comparison of post-operative complications in modified radical mastectomy patients with full suction and compression bandage versus half suction and non-compression bandage. International Surgery Journal, 7(5), 1541. https://doi.org/10.18203/2349-2902.isj20201866
- Sajjad, A., Kala, S., Gupta, A., & Varshney, A. (2020). Comparison of post-operative complications in modified radical mastectomy patients with full suction and compression bandage versus half suction and non-compression bandage. International Surgery Journal, 7(5), 1541. https://doi.org/10.18203/2349-2902.isj20201866
- Schuh, A. (2024). Compression dressing versus noncompressive transparent eye shield after ptosis surgery. Plastic and Reconstructive Surgery Global Open, 12(1), e5548. https://doi.org/10.1097/gox.0000000000005548
- Sonter, J., Tehan, P., & Chuter, V. (2017). Toe brachial index measured by automated device compared to duplex ultrasonography for detecting peripheral arterial disease in older people. Vascular, 25(6), 612-617. https://doi.org/10.1177/1708538117705293
- Valle, W. (2024). Utility of toe-brachial index. Journal for Vascular Ultrasound, 48(2), 83-87. https://doi.org/10.1177/15443167241249246
- Wang, F., Zhang, W., Li, H., Chen, X., Feng, S., & Mei, Z. (2022). How effective are nano-based dressings in diabetic wound healing? a comprehensive review of literature. International Journal of Nanomedicine, Volume 17, 2097-2119. https://doi.org/10.2147/ijn.s361282
- Wu, S., Crews, R., Skratsky, M., Overstreet, J., Yalla, S., Winder, M., … & Andersen, C. (2017). Control of lower extremity edema in patients with diabetes: double blind randomized controlled trial assessing the efficacy of mild compression diabetic socks. Diabetes Research and Clinical Practice, 127, 35-43. https://doi.org/10.1016/j.diabres.2017.02.025
- Zobel, E., Scholten, B., Reinhard, H., Persson, F., Hansen, T., Parving, H., … & Rossing, P. (2017). Toe–brachial index as a predictor of cardiovascular disease and all-cause mortality in people with type 2 diabetes and microalbuminuria. Diabetologia, 60(10), 1883-1891. https://doi.org/10.1007/s00125-017-4344-x